| Cope reaction | |
|---|---|
| Named after | Arthur C. Cope |
| Reaction type | Elimination reaction |
| Identifiers | |
| Organic Chemistry Portal | cope-elimination |
| RSC ontology ID | RXNO:0000539 |
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is the elimination reaction of an N-oxide to an alkene and a hydroxylamine.[1][2][3][4]

Typically, the amine oxide is prepared from the corresponding amine with a peroxy acid or comparable oxidant. The actual elimination requires just heat.
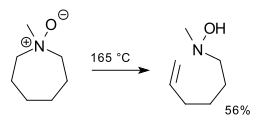
Illustrative is a synthesis of methylenecyclohexane:[5]

YouTube Encyclopedic
-
1/3Views:3 9793 8112 746
-
Cope Reaction | Intramolecular elimination -JEE|Medical|CBSE|Hindi
-
Hofmann and Cope Elimination Reaction Mechanism - E2, Syn vs Anti Stereochemistry, Organic Chemistry
-
Amine to Alkene - Cope Elimination Reaction Mechanism
Transcription
The reaction proceeds through the Ei pathway, with an intramolecular, cyclic 5-membered transition state.[1] Consequently, the elimination product is always syn and rarely occurs with 6-membered rings. (Rings with 5 or 7 or more members undergo the reaction just fine.)[6][7][8]
This organic reaction is closely related to the Hofmann elimination,[2] but the base is a part of the leaving group. Sulfoxides can undergo an essentially identical reaction to produce sulfenic acids, which is important in the antioxidant chemistry of garlic and other alliums. Selenoxides likewise undergo selenoxide eliminations.
Reverse reaction
The reverse or retro-Cope elimination has been reported, in which an N,N-disubstituted hydroxylamine reacts with an alkene to form a tertiary N-oxide.[9][10] The reaction is a form of hydroamination and can be extended to the use of unsubstituted hydroxylamine, in which case oximes are produced.[11]
References
- ^ Cope, Arthur C.; Foster, Theodore T.; Towle, Philip H. (1949). "Thermal Decomposition of Amine Oxides to Olefins and Dialkylhydroxylamines". Journal of the American Chemical Society. 71 (12): 3932–3935. doi:10.1021/ja01180a014.
- ^ Cope, Arthur C.; Towle, Philip H. (1949). "Rearrangement of Allyldialkylamine Oxides and Benzyldimethylamine Oxide". Journal of the American Chemical Society. 71 (10): 3423–3428. doi:10.1021/ja01178a048.
- ^ Cope, Arthur C.; Pike, Roscoe A.; Spencer, Claude F. (1953). "Cyclic Polyolefins. XXVII. cis- and trans-Cycloöctene from N,N-Dimethylcycloöctylamine". Journal of the American Chemical Society. 75 (13): 3212–3215. doi:10.1021/ja01109a049.
- ^ Peter C. Astles; Simon V. Mortlock; Eric J. Thomas (1991). "The Cope Elimination, Sulfoxide Elimination and Related Thermal Reactions". Comprehensive Organic Synthesis. Vol. 6. pp. 1011–1039. doi:10.1016/B978-0-08-052349-1.00178-5. ISBN 978-0-08-052349-1.
- ^ Cope, Arthur C.; Ciganek, Engelbert (1963). "Methylenecyclohexane and N,N-Dimethylhydroxylamine Hydrochloride". Organic Syntheses. 4: 612. doi:10.15227/orgsyn.039.0040.
- ^ March, Jerry; Smith, Michael B. (2007). March's advanced organic chemistry: reactions, mechanisms, and structure (6th. ed.). Wiley-Interscience. p. 1525. ISBN 978-0-471-72091-1.
- ^ Amine Oxides. VIII. Medium-sized Cyclic Olefins from Amine Oxides and Quaternary Ammonium Hydroxides Arthur C. Cope, Engelbert Ciganek, Charles F. Howell, Edward E. Schweizer J. Am. Chem. Soc., 1960, 82 (17), pp 4663–4669 doi:10.1021/ja01502a053
- ^ Amine Oxides. VII. The Thermal Decomposition of the N-Oxides of N-Methylazacycloalkanes Arthur C. Cope, Norman A. LeBel; J. Am. Chem. Soc.; 1960; 82(17); 4656-4662. doi:10.1021/ja01502a052
- ^ Ciganek, Engelbert; Read, John M.; Calabrese, Joseph C. (September 1995). "Reverse Cope elimination reactions. 1. Mechanism and scope". The Journal of Organic Chemistry. 60 (18): 5795–5802. doi:10.1021/jo00123a013.
- ^ Ciganek, Engelbert (September 1995). "Reverse Cope elimination reactions. 2. Application to synthesis". The Journal of Organic Chemistry. 60 (18): 5803–5807. doi:10.1021/jo00123a014.
- ^ Beauchemin, André M.; Moran, Joseph; Lebrun, Marie-Eve; Séguin, Catherine; Dimitrijevic, Elena; Zhang, Lili; Gorelsky, Serge I. (8 February 2008). "Intermolecular Cope-Type Hydroamination of Alkenes and Alkynes". Angewandte Chemie. 120 (8): 1432–1435. Bibcode:2008AngCh.120.1432B. doi:10.1002/ange.200703495.
