| Pomeranz–Fritsch reaction | |
|---|---|
| Named after | Cäsar Pomeranz Paul Fritsch |
| Reaction type | Ring forming reaction |
The Pomeranz–Fritsch reaction, also named Pomeranz–Fritsch cyclization, is a named reaction in organic chemistry. It is named after Paul Fritsch (1859–1913) and Cäsar Pomeranz (1860–1926).[1][2] In general it is a synthesis of isoquinoline.[2][3][4]
YouTube Encyclopedic
-
1/3Views:4 1792 9462 325
-
📚 Synthesis of Isoquinoline / Pictet Spengler Reaction mechanism application/ UGC CSIR NET GATE JAM
-
B.Sc. III Year | Heterocyclic compounds | Isoquinoline | Chemical reaction | आइसोक्विनोलिन
-
Benzopyridines - Quinolines And Isoquinolines
Transcription
General reaction scheme
The reaction below shows the acid-promoted synthesis of isoquinoline from benzaldehyde and a 2,2-dialkoxyethylamine.[5]
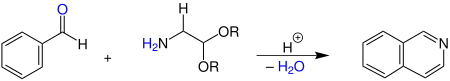
Various alkyl groups, e.g. methyl and ethyl groups, can be used as substituent R.
In the archetypical reaction sulfuric acid was used as proton donor, but Lewis acids such as trifluoroacetic anhydride and lanthanide triflates have been used occasionally.[1][2][4] Later, a wide range of diverse isoquinolines were successfully prepared.[4]
Reaction mechanism
A possible mechanism is depicted below:[5]

First the benzalaminoacetal 1 is built by the condensation of benzaldehyde and a 2,2-dialkoxyethylamine. After the condensation a hydrogen-atom is added to one of the alkoxy groups. Subsequently, an alcohol is removed. Next, the compound 2 is built. After that a second hydrogen-atom is added to the compound. In the last step a second alcohol is removed and the bicyclic system becomes aromatic.
Applications
The Pomeranz–Fritsch reaction has general application in the preparation of isoquinoline derivatives.
Isoquinolines find many applications, including:[3][4]
- topical anesthetics such as dimethisoquin:
- vasodilators, a well-known example, papaverine, shown below.
See also
References
- ^ a b Pomeranz, C. (December 1893). "Uber eine neue Isochinolinsynthese". Monatshefte für Chemie. 14 (1): 116–119. doi:10.1007/BF01517862. S2CID 95923801.
- ^ a b c Fritsch, Paul (January 1893). "Synthesen in der Isocumarin- und Isochinolinreihe". Berichte der Deutschen Chemischen Gesellschaft. 26 (1): 419–422. doi:10.1002/cber.18930260191.
- ^ a b Wang, Zerong (2009). Comprehensive organic name reactions and reagents. Hoboken, N.J.: John Wiley. pp. 2256–2259. ISBN 978-0-471-70450-8.
- ^ a b c d Kürti, László; Czakó, Barbara (2007). Strategic applications of named reactions in organic synthesis : background and detailed mechanisms ; 250 named reactions (Pbk. ed., [Nachdr.]. ed.). Amsterdam [u.a.]: Elsevier Academic Press. pp. 358–359. ISBN 978-0-12-429785-2.
- ^ a b Li, Jie Jack (2006). Name reactions : a collection of detailed reaction mechanisms ; [more than 300 reactions] (3., expanded ed.). Berlin [u.a.]: Springer. pp. 472–474. ISBN 978-3-540-30030-4.


