| Gattermann formylation | |
|---|---|
| Named after | Ludwig Gattermann |
| Reaction type | Substitution reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000139 |
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as aluminium chloride (AlCl3).[1] It is named for the German chemist Ludwig Gattermann[2] and is similar to the Friedel–Crafts reaction.
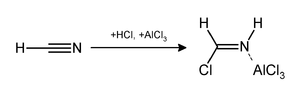

Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide.[3]
The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide.[4] Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN.[5] The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst in-situ. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene.[6]
YouTube Encyclopedic
-
1/3Views:26 34410 8791 679
-
Gatterman Koch Reaction
-
Gattermann Reaction Mechanism (Gattermann aldehyde synthesis Reaction)
-
Gattermann Formylation Reaction mechanism with scope and application JAM TIFR CSIR NET
Transcription
Gattermann–Koch reaction
| Gattermann–Koch formylation | |
|---|---|
| Named after | Ludwig Gattermann Julius Arnold Koch |
| Reaction type | Substitution reaction |
The Gattermann–Koch reaction, named after the German chemists Ludwig Gattermann and Julius Arnold Koch,[7] is a variant of the Gattermann reaction in which carbon monoxide (CO) is used instead of hydrogen cyanide.[8]
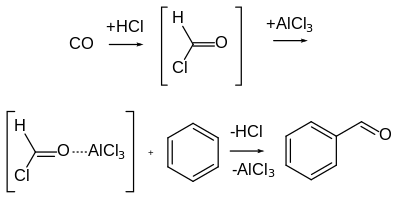
Unlike the Gattermann reaction, this reaction is not applicable to phenol and phenol ether substrates.[5] Although the highly unstable formyl chloride was initially postulated as an intermediate, formyl cation (i.e., protonated carbon monoxide), [HCO]+, is now thought to be react directly with the arene without the initial formation of formyl chloride.[9] Additionally, when zinc chloride is used as the Lewis acid instead of aluminum chloride for example, or when the carbon monoxide is not used at high pressure, the presence of traces of copper(I) chloride or nickel(II) chloride co-catalyst is often necessary. The transition metal co-catalyst may server as a "carrier" by first forming reacting with CO to form a carbonyl complex, which is then transformed into the active electrophile.[10]
See also
References
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 725, ISBN 978-0-471-72091-1
- ^ Gattermann, L.; Berchelmann, W. (1898). "Synthese aromatischer Oxyaldehyde". Berichte der deutschen chemischen Gesellschaft. 31 (2): 1765–1769. doi:10.1002/cber.18980310281.
- ^ Karrer, P. (1919). "Über Oxycarbonylverbindungen I. Eine neue Synthese von" [Hydroxycarbonyl compounds. I. A new synthesis of hydroxyaldehydes]. Helvetica Chimica Acta (in German). 2 (1): 89–94. doi:10.1002/hlca.19190020109.
- ^ Adams R.; Levine, I. (1923). "Simplification of the Gattermann Synthesis of Hydroxy Aldehydes". J. Am. Chem. Soc. 45 (10): 2373–77. doi:10.1021/ja01663a020.
- ^ a b Adams, Roger (1957). Organic Reactions, Volume 9. New York: John Wiley & Sons, Inc. pp. 38 & 53–54. doi:10.1002/0471264180.or009.02. ISBN 9780471007265.
- ^ Fuson, R. C.; Horning, E. C.; Rowland, S. P.; Ward, M. L. (1955). "Mesitaldehyde". Organic Syntheses. doi:10.15227/orgsyn.023.0057; Collected Volumes, vol. 3, p. 549.
- ^ Gattermann, L.; Koch, J. A. (1897). "Eine Synthese aromatischer Aldehyde". Chemische Berichte. 30 (2): 1622–1624. doi:10.1002/cber.18970300288.
- ^ Li, Jie Jack (2003). Name Reactions: A Collection of Detailed Reaction Mechanisms (available on Google Books) (2nd ed.). Springer. p. 157. ISBN 3-540-40203-9.
- ^ Kurti, Laszlo. (2005). Strategic Applications of Named Reactions in Organic Synthesis : Background and Detailed Mechanisms. Czako, Barbara. Burlington: Elsevier Science. ISBN 978-0-08-057541-4. OCLC 850164343.
- ^ Dilke, M. H.; Eley, D. D. (1949). "550. The Gattermann–Koch reaction. Part II. Reaction kinetics". J. Chem. Soc.: 2613–2620. doi:10.1039/JR9490002613. ISSN 0368-1769.
