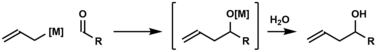
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol.[1] The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.[2]
Enantioselective versions
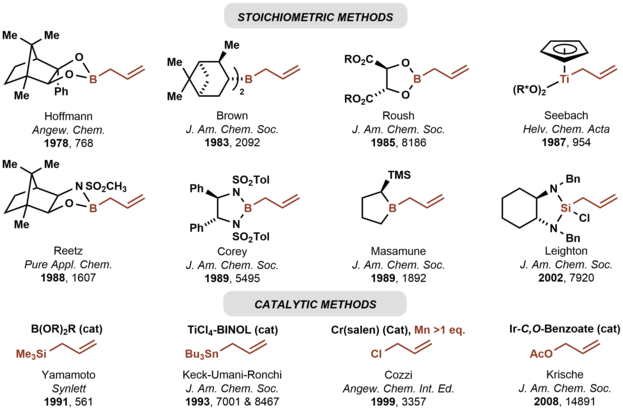
In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor.[3][4] Such methods utilize preformed allyl metal reagents. The approach is well developed using allyl boranes[5]
(13)

As illustrated by the Keck allylation,[6] catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by organostannane additions.[7]
Allylic boronate and -borane reagents have also been developed for enantioselective addition to carbonyls—in this class of reactions, the allylic boron reagent confers stereochemical control[5]
(13)

Catalysis
In 1991, Yamamoto disclosed the first catalytic enantioselective method for carbonyl allylation, which employed a chiral boron Lewis acid-catalyst in combination with allyltrimethylsilane.[8] Numerous other catalytic enantioselective methods for carbonyl allylation followed.[9][6] Catalytic variants of the Nozaki-Hiyama-Kishi reaction represent an alternative method for asymmetric carbonyl allylation, but stoichiometric metallic reductants are required.[10]
Whereas the aforementioned asymmetric carbonyl allylations rely on preformed allylmetal reagents, the Krische allylation exploits allyl acetate for enantioselective carbonyl allylation.[11] Selected methods for asymmetric carbonyl allylation are summarized below.
Use in total synthesis
Carbonyl allylation has been employed in the synthesis of polyketide natural products and other oxygenated molecules with a contiguous array of stereocenters. For example, allylstannanation of a threose-derived aldehyde affords the macrolide antascomicin B, which structurally resembles FK506 and rapamycin, and is a potent binder of FKBP12.[12] The Krische allylation was used to prepare the polyketide (+)-SCH 351448, a macrodiolide ionophore bearing 14 stereogenic centers.[13]

Older primary literature
- Brown, Herbert C.; Jadhav, Prabhakar K. (April 1983). "Asymmetric carbon-carbon bond formation via .beta.-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities". Journal of the American Chemical Society. 105 (7): 2092–2093. doi:10.1021/ja00345a085. ISSN 0002-7863.
- Hayashi, Tamio; Konishi, Mitsuo; Kumada, Makoto (1982-09-01). "Optically active allylsilanes. 2. High stereoselectivity in asymmetric reaction with aldehydes producing homoallylic alcohols". Journal of the American Chemical Society. 104 (18): 4963–4965. doi:10.1021/ja00382a046.
- Roush, William R.; Walts, Alan E.; Hoong, Lee K. (1985-12-01). "Diastereo- and enantioselective aldehyde addition reactions of 2-allyl-1,3,2-dioxaborolane-4,5-dicarboxylic esters, a useful class of tartrate ester modified allylboronates". Journal of the American Chemical Society. 107 (26): 8186–8190. doi:10.1021/ja00312a062.
- Brown, Herbert C.; Jadhav, Prabhakar K. (April 1983). "Asymmetric carbon-carbon bond formation via .beta.-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities". Journal of the American Chemical Society. 105 (7): 2092–2093. doi:10.1021/ja00345a085. ISSN 0002-7863.
- Kinnaird, James W. A.; Ng, Pui Yee; Kubota, Katsumi; Wang, Xiaolun; Leighton, James L. (2002-07-01). "Strained Silacycles in Organic Synthesis: A New Reagent for the Enantioselective Allylation of Aldehydes". Journal of the American Chemical Society. 124 (27): 7920–7921. doi:10.1021/ja0264908. ISSN 0002-7863. PMID 12095334.
- Short, Robert P.; Masamune, Satoru (March 1989). "Asymmetric allylboration with B-allyl-2-(trimethylsilyl)borolane". Journal of the American Chemical Society. 111 (5): 1892–1894. doi:10.1021/ja00187a061. ISSN 0002-7863.
- Corey, E. J.; Yu, Chan Mo; Kim, Sung Soo (July 1989). "A practical and efficient method for enantioselective allylation of aldehydes". Journal of the American Chemical Society. 111 (14): 5495–5496. doi:10.1021/ja00196a082. ISSN 0002-7863.
References
- ^ Yus, Miguel; González-Gómez, José C.; Foubelo, Francisco (2011). "Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines". Chemical Reviews. 111 (12): 7774–7854. doi:10.1021/cr1004474. PMID 21923136.
- ^ Michael; Saytzeff, Alexander (1877). "Synthese des Allyldimethylcarbinols". Justus Liebigs Annalen der Chemie. 185 (2–3): 151–169. doi:10.1002/jlac.18771850204. ISSN 1099-0690.
- ^ Herold, Thomas; Hoffmann, Reinhard W. (1978-10-01). "Enantioselective Synthesis of Homoallyl Alcohols via Chiral Allylboronic Esters". Angewandte Chemie International Edition in English. 17 (10): 768–769. doi:10.1002/anie.197807682.
- ^ Hoffmann, Reinhard W.; Herold, Thomas (1981-01-01). "Stereoselektive Synthese von Alkoholen, VII1) Optisch aktive Homoallylalkohole durch Addition chiraler Boronsäureester an Aldehyde". Chemische Berichte. 114 (1): 375–383. doi:10.1002/cber.19811140139.
- ^ a b Denmark, S. E.; Almstead, N. G. In Modern Carbonyl Chemistry; Otera, J., Ed.; Wiley-VCH: Weinheim, 2000; Chapter 10.
- ^ a b Keck, Gary E.; Tarbet, Kenneth H.; Geraci, Leo S. (1993-09-01). "Catalytic asymmetric allylation of aldehydes". Journal of the American Chemical Society. 115 (18): 8467–8468. doi:10.1021/ja00071a074.
- ^ Denmark, Scott E.; Fu, Jiping (2003-08-01). "Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones". Chemical Reviews. 103 (8): 2763–2794. doi:10.1021/cr020050h. ISSN 0009-2665. PMID 12914480.
- ^ Furuta, Kyoji; Mouri, Makoto; Yamamoto, Hisashi (1991-01-01). "Chiral (Acyloxy)borane Catalyzed Asymmetric Allylation of Aldehydes". Synlett. 1991 (8): 561–562. doi:10.1055/s-1991-20797.
- ^ Costa, Anna Luisa; Piazza, Maria Giulia; Tagliavini, Emilio; Trombini, Claudio; Umani-Ronchi, Achille (1993-07-01). "Catalytic asymmetric synthesis of homoallylic alcohols". Journal of the American Chemical Society. 115 (15): 7001–7002. doi:10.1021/ja00068a079.
- ^ Hargaden, Gráinne C.; Guiry, Patrick J. (2007-11-05). "The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction". Advanced Synthesis & Catalysis. 349 (16): 2407–2424. doi:10.1002/adsc.200700324.
- ^ Kim, In Su; Ngai, Ming-Yu; Krische, Michael J. (2008-11-05). "Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level via Transfer Hydrogenative Coupling of Allyl Acetate: Departure from Chirally Modified Allyl Metal Reagents in Carbonyl Addition". Journal of the American Chemical Society. 130 (44): 14891–14899. doi:10.1021/ja805722e. ISSN 0002-7863. PMC 2890235. PMID 18841896.
- ^ Brittain, Dominic E. A.; Griffiths-Jones, Charlotte M.; Linder, Michael R.; Smith, Martin D.; McCusker, Catherine; Barlow, Jaqueline S.; Akiyama, Ryo; Yasuda, Kosuke; Ley, Steven V. (2005). "Total Synthesis of Antascomicin B". Angewandte Chemie International Edition. 44 (18): 2732–2737. doi:10.1002/anie.200500174. ISSN 1521-3773. PMID 15806607.
- ^ Wang, Gang; Krische, Michael J. (2016-07-06). "Total Synthesis of (+)-SCH 351448: Efficiency via Chemoselectivity and Redox-Economy Powered by Metal Catalysis". Journal of the American Chemical Society. 138 (26): 8088–8091. doi:10.1021/jacs.6b04917. ISSN 0002-7863. PMC 4935581. PMID 27337561.
