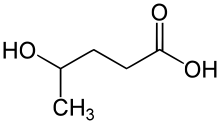
γ-Hydroxyvaleric acid (GHV ), also known as 4-methyl-GHB , is a designer drug related to γ-hydroxybutyric acid (GHB). It is sometimes seen on the grey market as a legal alternative to GHB, but with lower potency and higher toxicity,[2] [3]
γ-Valerolactone (GVL) acts as a prodrug to GHV, analogously to how γ-butyrolactone (GBL) is a prodrug to GHB.[4]
See also References
^ "GHB and Analogues: Fast Facts" . National Drug Intelligence Center . Retrieved July 5, 2023 .{{cite web }}: CS1 maint: bot: original URL status unknown (link )^ Carter LP, Chen W, Wu H, Mehta AK, Hernandez RJ, Ticku MK, et al. (April 2005). "Comparison of the behavioral effects of gamma-hydroxybutyric acid (GHB) and its 4-methyl-substituted analog, gamma-hydroxyvaleric acid (GHV)". Drug and Alcohol Dependence . 78 (1): 91–99. doi :10.1016/j.drugalcdep.2004.10.002 . PMID 15769562 . ^ Smith F (31 December 2004). Handbook of Forensic Drug Analysis ISBN 978-0-08-047289-8 ^ Andresen-Streichert H, Jungen H, Gehl A, Müller A, Iwersen-Bergmann S (May 2013). "Uptake of gamma-valerolactone--detection of gamma-hydroxyvaleric acid in human urine samples" . Journal of Analytical Toxicology . 37 (4): 250–254. doi :10.1093/jat/bkt013 PMID 23486087 .
Ionotropic
GABAA Tooltip γ-Aminobutyric acid A receptor
Positive modulators (abridged; see here for a full list): α-EMTBLAlcohols (e.g., drinking alcohol , 2M2B )Anabolic steroids Avermectins (e.g., ivermectin )Barbiturates (e.g., phenobarbital )Benzodiazepines (e.g., diazepam )Bromide compounds (e.g., potassium bromide )Carbamates (e.g., meprobamate )Carbamazepine Chloralose Chlormezanone Clomethiazole Dihydroergolines (e.g., ergoloid (dihydroergotoxine) )Etazepine Etifoxine Fenamates (e.g., mefenamic acid )Flavonoids (e.g., apigenin , hispidulin )Fluoxetine Flupirtine Imidazoles (e.g., etomidate )Kava constituents (e.g., kavain )Lanthanum Loreclezole Monastrol Neuroactive steroids (e.g., allopregnanolone , cholesterol , THDOC )Niacin Niacinamide Nonbenzodiazepines (e.g., β-carbolines (e.g., abecarnil ), cyclopyrrolones (e.g., zopiclone ), imidazopyridines (e.g., zolpidem ), pyrazolopyrimidines (e.g., zaleplon ))Norfluoxetine Petrichloral Phenols (e.g., propofol )Phenytoin Piperidinediones (e.g., glutethimide )Propanidid Pyrazolopyridines (e.g., etazolate )Quinazolinones (e.g., methaqualone )Retigabine (ezogabine) ROD-188 Skullcap constituents (e.g., baicalin )Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) )
Topiramate Valerian constituents (e.g., valerenic acid )Volatiles /gases (e.g., chloral hydrate , chloroform , diethyl ether , paraldehyde , sevoflurane )Negative modulators: 1,3M1B3M2B
11-Ketoprogesterone 17-Phenylandrostenol α3IA α5IA (LS-193,268) β-CCB
β-CCE
β-CCM
β-CCP
β-EMGBL
Anabolic steroids Amiloride Anisatin β-Lactams (e.g., penicillins , cephalosporins , carbapenems )Basmisanil Bemegride Bicyclic phosphates (TBPS , TBPO , IPTBO )BIDN Bilobalide Bupropion CHEB Chlorophenylsilatrane Cicutoxin Cloflubicyne Cyclothiazide DHEA DHEA-S Dieldrin (+)-DMBB DMCM DMPC
EBOB Etbicyphat
FG-7142 (ZK-31906) Fiproles (e.g., fipronil )
Flavonoids (e.g., amentoflavone , oroxylin A )Flumazenil Fluoroquinolones (e.g., ciprofloxacin )Flurothyl Furosemide Golexanolone Iomazenil (123 I) IPTBO Isopregnanolone (sepranolone) L-655,708 Laudanosine Lindane MaxiPost
Morphine Morphine-3-glucuronide MRK-016 Naloxone Naltrexone Nicardipine Nonsteroidal antiandrogens (e.g., apalutamide , bicalutamide , enzalutamide , flutamide , nilutamide )Oenanthotoxin Pentylenetetrazol (pentetrazol) Phenylsilatrane Picrotoxin (i.e., picrotin , picrotoxinin and dihydropicrotoxinin )Pregnenolone sulfate Propybicyphat
PWZ-029 Radequinil Ro 15-4513 Ro 19-4603 RO4882224
RO4938581
Sarmazenil SCS
Suritozole TB-21007 TBOB
TBPS TCS-1105
Terbequinil TETS Thujone U-93631 Zinc ZK-93426 GABAA -ρ Tooltip γ-Aminobutyric acid A-rho receptor
Metabotropic
GABAB Tooltip γ-Aminobutyric acid B receptor
Negative modulators: Compound 14
Receptor (ligands )
GHBR Tooltip GHB receptor GABAB Tooltip γ-Aminobutyric acid B receptor
Transporter (blockers )
MCTs Tooltip Monocarboxylate transporters SMCTs Tooltip Sodium-coupled monocarboxylate transporters VIATT Tooltip Vesicular inhibitory amino acid transporter
Enzyme (inhibitors )
SSR Tooltip Succinic semialdehyde reductase GHBDH Tooltip 4-Hydroxybutyrate dehydrogenase HOT Tooltip Hydroxyacid-oxoacid transhydrogenase ADH Tooltip Alcohol dehydrogenase ALDH Tooltip Aldehyde dehydrogenase
This page was last edited on 10 March 2024, at 21:19