 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
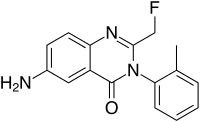
| Formula | C16H14FN3O |
| Molar mass | 283.306 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Afloqualone (Arofuto) is a quinazolinone family GABAergic drug and is an analogue of methaqualone developed in the 1970s by a team at Tanabe Seiyaku.[1] It has sedative and muscle-relaxant effects resulting from its agonist activity at the β subtype of the GABAa receptor[2] and has had some clinical use, although it causes photosensitization as a side-effect that can cause skin problems such as dermatitis.[3]
See also
References
- ^ US 3966731, Inoue I, Oine T, Yamada Y, Tani J, Ishida R, Ochiai T, "2-Fluoromethyl-3-o-tolyl-6-amino-4(3H)-quinazolinone", issued 29 June 1976, assigned to Tanabe Seiyaku Co Ltd
- ^ Ochiai T, Ishida R (June 1982). "Pharmacological studies on 6-amino-2-fluoromethyl-3-(O-tolyl)-4(3H)-quinazolinone (afloqualone), a new centrally acting muscle relaxant. (II) Effects on the spinal reflex potential and the rigidity". Japanese Journal of Pharmacology. 32 (3): 427–38. doi:10.1254/jjp.32.427. PMID 7109348.
- ^ Ishikawa T, Kamide R, Niimura M (June 1994). "Photoleukomelanodermatitis (Kobori) induced by afloqualone". The Journal of Dermatology. 21 (6): 430–3. doi:10.1111/j.1346-8138.1994.tb01768.x. PMID 8064007. S2CID 7486566.
