 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.002 |
| Chemical and physical data | |
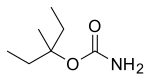
| Formula | C7H15NO2 |
| Molar mass | 145.202 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Emylcamate (marketed as Striatran by Merck) is an anxiolytic and muscle relaxant. It was patented in the US in 1961 (US Patent 2,972,564) and advertised for the treatment of anxiety and tension. It was claimed to be superior to meprobamate, which would eventually replace emylcamate.
A study of the drug's effects in mice was done in 1959. It concluded that at 50 mg/kg emylcamate gave a 63% decrease in motor activity compared with meprobamate's 32% decrease, a doubling in effective potency. The therapeutic index in mice was also established:
| Meprobamate | Emylcamate | Effect |
|---|---|---|
| 175 | 123 | ED50 (mg/kg) |
| 600 | 550 | LD50 (mg/kg) |
| 3.4 | 4.4 | Therapeutic index |
Emylcamate also has a faster intra-parenteral onset than meprobamate, 3 minutes compared with 35.[2]
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Melander B (1959). "Emylcamate, A Potent Tranquillizing Relaxant". Journal of Medicinal and Pharmaceutical Chemistry. 1 (5): 443–457. doi:10.1021/jm50006a003.
Further reading
- Shorter E (August 2004). "Olhando para trás: um novo caminho possível para a descoberta de drogas em psicofarmacologia" [Looking back: a new possible path for drug discovery in psychopharmacology.]. Revista de Psiquiatria do Rio Grande do Sul (in Portuguese). 26 (2): 196–203. doi:10.1590/S0101-81082004000200009.
External links
- "Emylcamate". The Comparative Toxicogenomics Database.
- "Emylcamate". BIAM (in French). Archived from the original on 2022-09-26. Retrieved 2005-12-22.
