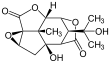
Picrotoxinin (left) and picrotin (right) | |||
| Clinical data | |||
|---|---|---|---|
| ATC code |
| ||
| Identifiers | |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.004.288 | ||
| Chemical and physical data | |||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812.[1] The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison).[2] A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.
Due to its interactions with the inhibitory neurotransmitter GABA, picrotoxin acts as a stimulant and convulsant. It mainly impacts the central nervous system, causing seizures and respiratory paralysis in high enough doses.
YouTube Encyclopedic
-
1/2Views:294 1382 434
-
The GABA receptor | How does it work?
-
Lindane
Transcription
And the last part here before we jump into these drugs is the structure and function of this GABA Chloride Ion Channel that we can see right here. So just taking a first look at it right, this is a GABA Chloride Ion Channel and the neurotransmitter is GABA. Now, I didn't mention this earlier because I didn't want to overwhelm you. but notice here, it's not just 1 GABA that needs to bind but actually 2 GABAs need to bind in order to let an ion through here. So I'm just going to write a neurotransmitter x 2. What is this receptor called? Well it's called the GABA A receptor and the reason it's called the GABA A receptor is due to the actual type of sub-units that comes through that comprises this channel and also, the ion that goes through it. And we'll talk about GABA B on the next slide. What is the ion here? Well, the ion is called Chloride. And so, what happens? Chloride comes into the cell and it makes the inside of the cell slightly more negative and the effect of that is an inhibitory post-synaptic potential. So, let's put these all in context because it's kind of confusing unless you see it all together. So let's just say here is my pre-synaptic neuron and let's say here's my post-synaptic neuron. Now, I'm going to draw this a little different than you might see it because we're going to add something to it here in a second. So what's going on here? Well, we've got a different color. So we have an action potential right. It comes down the cell. We have this voltage gated calcium channel, this allows calcium to come in right. And so, calcium comes in and it causes the fusion of these vesicles and what are these vesicles containing? They're containing GABA. And so, from this pre-synaptic neuron, these vesicles traverse across the synaptic cleft and they will bind to a receptor on the post-synaptic membrane. So let's just say here is the ion channel, here is the receptor. That's what we're looking at right here. That receptor is that one here. So here's step one, action potential comes. Two, calcium comes in. Three, we get the fusion GABA and four, it diffuses across. Five, it binds to the receptor and that's what we're seeing here. And so six would be the Chloride ions that are here coming into the post-synaptic neuron causing an inhibitory post-synaptic potential and so, that's what we see right here. Now, this isn't the only neuron that's interacting with this post-synaptic neuron right? We can also have an excitatory one that's synapsing here as well. So, I'm not going to draw everything like I did before but let's say we have all of these steps but instead of GABA coming here, let's say we have (man! it's crazy how nice colors look) let's say we have glutamate. That was a silly comment. And so, with glutamate right it also binds to its own receptor but in this case, let's say, it lets sodium in and now, we have an excitatory post-synaptic potential. And so, what this cell is doing is it well it says, okay, is there more positive or is there more negative? If there's more positive, is it enough to reach threshold? And if there is, then maybe I'll list another action potential here and here are these you know sodium channels on the axon that allow charge to come through. Remember anywhere you block along this path, well you can mess things up. So this can also be represented with a little diagram here. And so, when I say that the sedative-hypnotics have dose dependent CNS depressant effects, this will kind of explain why. So right off the bat, we can - let me give us, what's our color? Let's go back to orange. So here, let's say we have this excitatory stimulus. let's say this was glutamate. It was enough to reach threshold. We kick off. Boom! And all our non-action potential and we come back to resting membrane potential but now we get this inhibitory stimulus, okay so this let's say is GABA and this GABA caused a dip in this membrane potential. Now the next time this excitatory stimulus comes along glutamate, it is not enough to reach threshold and as a result, we get the CNS depression. The depression is a result of no more communication or decreased communication. And so, if this guy overwhelms here, we're not going to get this action potential and therefore, we're depressing the central nervous system. Makes sense? All right, so let's take a moment and look at the receptors for the different neurotransmitters and drugs here. So the first thing you should notice is we have GABA here, we have the benzodiazepines here and we have the barbs here. And so, these are all binding at distinct locations and if you can't tell from this kind of cross sectional view, maybe a top down view might be a little better So we have GABA which is the endogenous neurtransmitter and by convention, the endogenous neurotransmitter typically binds to the alpha sub-unit. Here we have our Benzodiazepines represented with a bz and this binds to the alpha and gamma sub-unit that we can kind of see right here. This was alpha, this was gamma. And here, we have our Barbs and our Barbs are binding to the beta and gamma sub-unit. So what's important here is that they're not the Barbs and the Benzos, they're not competing for the same binding site and as a result, they can have additive effects. Okay, it's important to know that they're not competing. Also, there's a lot of heterogeneity even within this GABA receptor. This GABA A receptor And in particular, notice that the benzos are binding here and this binding site actually has a name and we call it the bz 1 receptor and this is probably due to the fact that there's this alpha 1 sub-unit here. Now, the only reason I mentioned this, you don't need to memorize the exact molecular make-up of the but some of our newer hypnotics are very selective for this bz 1 receptor with this alpha 1 sub-unit. Newer hypnotics are selective and because of the location of these receptors, we can get these hypnotic effects which is making you sleepy without as much of the anxiolytics effects and that's kind of one of the benefits of these newer hypnotics. So you get more hypnotic effect with less sedative effect and we can see that right here. This is Ambien or Zolpidem. and the last point I want to make is not only do we have selectivity for you know particular binding sites but also for ions. So this is the GABA A chloride ion channel right but we also have a GABA B channel or a GABA B I guess potassium ion channel. GABA B. And so, unlike this which allows chloride to come into the cell, this GABA B lets potassium out. and the drug that interacts most with this receptor is Baclofen which is a spasmolytic and you would use that for muscle spasms and we probably won't talk about Baclofen again but it's just an important thing to know right here. Finally, one last point while we're looking here. We have this guy right here, Flumazenil. And notice where it is. It's right here for between the benzos and the newer hypnotics but nowhere near where the Barbs are and so, what this is, is actually it's an antagonist. I can't write it all out but let's write it here. And so, this is special and that is it can reverse the effects of overdoses from Benzos or Zolpidem and won't have any effect on the Barbs because they have different binding sites. So again, understanding the structure will really tell you the function of how this work and that my friends is sedative-hypnotics, the foundations. On a last little thing here, here are some questions that hopefully you should be able to answer having attentively watched these lectures. So it's learning the objectives with homework questions. Feel free to do these on your own. Subtitles by the Amara.org community
Chemical structure and synthesis
Picrotoxin is an equimolar mixture of two compounds, picrotoxinin (C15H16O6; CAS# 17617-45-7) and picrotin (C15H18O7; CAS# 21416-53-5).[3] Of the two compounds, picrotin is less active.[4]
Picrotoxin occurs naturally in the fruit of the Anamirta cocculus, a climbing plant from India and other parts of Southeast Asia. The plant is known for its large stems of white wood and sweetly-scented flowers. It produces small stone fruits, Cocculus indicus, which are typically dried.[citation needed]
Currently, there are as many as five total syntheses of picrotoxinin — one of which was published as recently as June 2020.[5] Most syntheses use carvone as a stereochemical template.
![Begin with methyl (1S,4S,5R,7R,8S,9R,10R,11R)-10-(acetyloxy)-7-hydroxy-11-methyl-3-oxo-9-(prop-1-en-2-yl)-4,5-bis[(trimethylsilyl)oxy]-2-oxatricyclo[5.3.1.04,11]undecane-8-carboxylate. (1) Intramolecular transesterification, releasing methyl acetate; then (2) deprotection of a trimethylsilyl-protected vicinal diol, followed by (3) reductive dehydration to an olefin, and (4) stereospecific epoxidation to a glycidic ester](http://upload.wikimedia.org/wikipedia/commons/thumb/9/96/Picrotoxinin_Synthesis.png/500px-Picrotoxinin_Synthesis.png)
In 1988, researchers from Tohoku University in Japan completed a total stereoselective synthesis of both (‑)‑picrotoxinin and (-)-picrotin beginning with (+)‑5β‑hydroxycarvone. In this synthesis, eight asymmetric centers were stereoselectively prepared on a cis-fused hydrindane ring system using several different reactions: a Claisen rearrangement to introduce the quaternary center, an organoselenium-mediated reduction of an epoxy ketone, and a stereospecific construction of a glycidic ester.[7]
The June 2020 synthesis instead employed the quick formation of the polycyclic core, followed by the manipulation of oxidation states of key carbon atoms in order to produce the target molecule.[5]
Some research suggests that picritoxin can be made by the cyclofunctionalization of cycloalkenyl systems. Under kinetically controlled conditions, this process generally results in exo cyclization and forms bridged ring systems like those found in picrotoxin.[8]
Several techniques have been developed to isolate picrotoxinin and picrotin individually. Reaction with the nearby cis alcohol is the key obstruction, and can be inhibited by pretreatment (protection) with trifluoroacetic anhydride in pyridine:[9]

Picrotoxin has also been used as a starting material in several synthetic processes, including the creation of dl-picrotoxadiene, which retains certain features of the picrotoxin skeleton.[10]
Mechanism of action
Some crustacean muscle fibers have excitatory and inhibitory innervation. Picrotoxin blocks inhibition.[11] Two different but related theories have been proposed for the mechanism by which picrotoxin acts on synapses. One theory is that it acts as a non-competitive channel blocker for GABAA receptor chloride channels,[12] specifically the gamma-aminobutyric acid-activated chloride ionophore.[13] A 2006 study found that, while not structurally similar to GABA, picrotoxin prevents ion flow through the chloride channels activated by GABA. It likely acts within the ion channels themselves, rather than at GABA recognition sites. Because it inhibits channels activated by GABA, GABA-enhancing drugs like barbiturates and benzodiazepines can be used as an antidote.[14]
Other research suggests that the toxin acts instead as a non-competitive antagonist, or inhibitor, for GABA receptors. A study by Newland and Cull-Candy found that, in high enough concentrations, picrotoxin reduced the amplitude of GABA currents. Their data indicated that it was unlikely that picrotoxin acted simply as a voltage-gated channel blocker, although it did reduce the frequency of channel openings. Rather, they found that picrotoxin “binds preferentially to an agonist bound form of the receptor.” This means that, even in the presence of low concentrations of picrotoxin, the response of neurons to GABA is reduced.[15]
Toxicity
Picrotoxin acts as a central nervous system and respiratory stimulant. It is extremely toxic to fish and humans, as well as rodents and other mammals. According to the Register of Toxic Effects of Chemical Substances, the LDLo, or lowest reported lethal dose, is 0.357 mg/kg. Symptoms of picrotoxin poisoning include coughing, difficulty breathing, headache, dizziness, confusion, gastro-intestinal distress, nausea or vomiting, and changes in heart rate and blood pressure. Although especially dangerous if swallowed, systemic effects can also result from inhalation or absorption into the blood stream through lesions in the skin.[16] Picrotoxin also acts as a convulsant. In larger doses, it has been found to induce clonic seizures or cardiac dysrhythmias, with especially high doses ultimately proving fatal, typically due to respiratory paralysis.[17]
Clinical applications and other uses
Due to its toxicity, picrotoxin is now most commonly used as a research tool. However, due to its antagonist effect on GABA receptors, it has been used as a central nervous system stimulant. It was also previously used as an antidote for poisoning by CNS depressants, especially barbiturates.[18]
Although not commonly used, picrotoxin is effective as both a pesticide and a pediculicide. In the 19th century, it was used in the preparation of hard multum, which was added to beer to make it more intoxicating. This preparation has since been outlawed.[19][20]
Despite its potential toxicity to mammals in large enough doses, picrotoxin is also sometimes used as a performance enhancer in horses. It is classified as an illegal "Class I substance" by the American Quarter Horse Association. Substances that are classified as “Class I” are likely to affect performance and have no therapeutic use in equine medicine.[21] In 2010, quarter horse trainer Robert Dimitt was suspended after his horse, Stoli Signature, tested positive for the substance. As with humans, it is used to counteract barbiturate poisoning.[22]
See also
References
- ^ Boullay PF (1812). "Analyse chimique de la Coque du Levant, Menispermum cocculus". Bulletin de Pharmacie (in French). 4: 5–34.
Menispermum cocculus" has been renamed "Anamirta cocculus"
- ^ (Boullay, 1812), p. 31.
- ^ Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, et al. "Picrotoxin". DrugBank. Retrieved April 26, 2017.
- ^ Gammill R, Tulinsky J (1994). "The Chemistry and Pharmacology of GABAA and GABAB Ligands". Current Medicinal Chemistry. 1 (3): 242. Retrieved April 26, 2017.
- ^ a b Crossley SW, Tong G, Lambrecht MJ, Burdge HE, Shenvi RA (July 2020). "Synthesis of (-)-Picrotoxinin by Late-Stage Strong Bond Activation". Journal of the American Chemical Society. 142 (26): 11376–11381. doi:10.1021/jacs.0c05042. PMC 8011636. PMID 32573211.
- ^ Trost B, Krische MJ (1996). "Picrotoxinin". Journal of the American Chemical Society. 118: 233. doi:10.1021/ja953060r. Retrieved May 7, 2017.
- ^ Miyashita M, Suzuki T, Yoshikoshi A (May 1989). "Stereoselective total synthesis of (-)-picrotoxinin and (-)-picrotin". Journal of the American Chemical Society. 111 (10): 3728–3734. doi:10.1021/ja00192a035.
- ^ Trost B, Fleming I (1991). Comprehensive Organic Synthesis (Volume 4 ed.). Oxford, UK: Pergamon Press. p. 373. ISBN 9780080405957. Retrieved May 7, 2017.
- ^ Corey EJ, Pearce HL (1980). "Total Synthesis of Picrotin". Tetrahedron Letters. 21 (19): 1823–1824. doi:10.1016/s0040-4039(00)92789-8.
- ^ Conroy H (June 1952). "Picrotoxin. II. The Skeleton of Picrotoxinin. The Total Synthesis of dl-Picrotoxadiene". Journal of the American Chemical Society. 74 (12): 3046–3051. doi:10.1021/ja01132a028.
- ^ Van Der Kloot WG, Robbins J, Cooke IM (March 1958). "Blocking by picrotoxin of peripheral inhibition in crayfish". Science. 127 (3297): 521–522. Bibcode:1958Sci...127..521V. doi:10.1126/science.127.3297.521. PMID 13529017.
- ^ Rho JM, Donevan SD, Rogawski MA (December 1996). "Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons". The Journal of Physiology. 497 (2): 509–22. doi:10.1113/jphysiol.1996.sp021784. PMC 1161000. PMID 8961191.
- ^ Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, et al. "Picrotoxin". DrugBank. Retrieved April 26, 2017.
- ^ Olsen RW (April 2006). "Picrotoxin-like channel blockers of GABAA receptors". Proceedings of the National Academy of Sciences of the United States of America. 103 (16): 6081–2. Bibcode:2006PNAS..103.6081O. doi:10.1073/pnas.0601121103. PMC 1458832. PMID 16606858.
- ^ Newland CF, Cull-Candy SG (February 1992). "On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat". The Journal of Physiology. 447: 191–213. doi:10.1113/jphysiol.1992.sp018998. PMC 1176032. PMID 1317428.
- ^ "Picrotoxin" (PDF). Santa Cruz Biotechnology. Retrieved April 26, 2017.
- ^ "Picrotoxin". Toxnet. U.S. National Laboratory of Medicine. Retrieved April 26, 2017.
- ^ Nilsson E, Eyrich B (2009). "On treatment of barbiturate poisoning". Acta Medica Scandinavica. 137 (6): 381–9. doi:10.1111/j.0954-6820.1950.tb12129.x. PMID 15432128.
- ^ Böttger A, Vothknecht U, Bolle C, Wolf A (2018). "Plant-Derived Drugs Affecting Ion Channels". Lessons on Caffeine, Cannabis & Co: Plant-derived Drugs and their Interaction with Human Receptors. Learning Materials in Biosciences. p. 129. doi:10.1007/978-3-319-99546-5_8. ISBN 978-3-319-99545-8.
- ^ Bell J (1869). Report of the Committee on the Relations of Alcohol to Medicine. United States: Collins. p. 32.
- ^ "Uniform Classification Guidelines for Foreign Substances and Recommended Penalties and Model Rule" (PDF). Association of Racing Commissioners International, Inc. Retrieved April 26, 2017.
- ^ Lemoreaux P (September 2, 2017). "Two Quarter Horse trainers suspended for drug violations at Prairie Meadows". Daily Racing Form. Daily Racing Form. Retrieved April 26, 2017.
Further reading
- Ehrenberger K, Benkoe E, Felix D (1982). "Suppressive action of picrotoxin, a GABA antagonist, on labyrinthine spontaneous nystagmus and vertigo in man". Acta Oto-Laryngologica. 93 (1–6): 269–73. doi:10.3109/00016488209130882. PMID 7064710.
- Dupont L, Dideberg O, Lamotte-Brasseur J, Angenot L (1976). "Structure cristalline et moléculaire de la picrotoxine, C15H16O6·C15H18O7". Acta Crystallographica B (in French). 32 (11): 2987–2993. doi:10.1107/S0567740876009424. hdl:2268/31560.
- Olsen RW, DeLorey TM (1999). "GABA Receptor Physiology and Pharmacology". In Siegel GJ, Agranoff BW, Albers RW, et al. (eds.). Basic Neurochemistry: Molecular, Cellular and Medical Aspects (6th ed.). Philadelphia, PA, USA: Lippincott-Raven.