 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
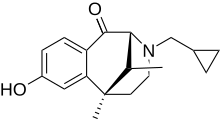
| Formula | C18H23NO2 |
| Molar mass | 285.387 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ketazocine (INN), also known as ketocyclazocine, is a benzomorphan derivative used in opioid receptor research. Ketazocine, for which the receptor is named, is an exogenous opioid that binds to the κ opioid receptor.[1]
Activation of this receptor is known to cause sleepiness, a decrease in pain sensation and (potentially) dysphoria, paranoia, and hallucinations. It also causes an increase in urine production because it inhibits the release of vasopressin. (Vasopressin is an endogenous substance that assists in regulating fluid and electrolyte balance in the body and decreases the amount of water released into the urine.)
Unlike other opioids, substances that only bind to the κ receptor theoretically do not depress the respiratory system.
The crystal structure of ketazocine was determined in 1983.[2]
See also
References
- ^ Leander JD (September 1982). "Effects of ketazocine, ethylketazocine and phenazocine on schedule-controlled behavior: antagonism by naloxone". Neuropharmacology. 21 (9): 923–8. doi:10.1016/0028-3908(82)90085-5. PMID 6128693. S2CID 38692038.
- ^ Verlinde CL, De Ranter CJ (1983). "(1S, 5R, 9R)-2-Cyclopropylmethyl-2'-hydroxy-5, 9-dimethyl-8-oxo-6, 7-benzomorphan hydrochloride monohydrate (ketazocine), C18H23NO2. HCl. H2O". Acta Crystallogr. C. 39 (12): 1703–1706. Bibcode:1983AcCrC..39.1703V. doi:10.1107/S0108270183009828.
| μ-opioid (MOR) |
| ||||
|---|---|---|---|---|---|
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
