 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H27N3O |
| Molar mass | 313.445 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
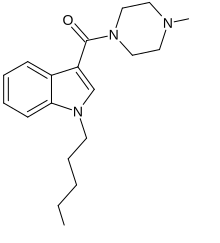
MEPIRAPIM is an indole-based cannabinoid which differs from JWH-018 by having a 4-methylpiperazine group in place of the naphthyl group[1] and has been used as an active ingredient in synthetic cannabis products. It was first identified in Japan in 2013, alongside FUBIMINA.[2] MEPIRAPIM acts as a T-type calcium channel inhibitor and is only minimally active at the central CB1 receptor.[3]
Legality
Sweden's public health agency suggested to classify MEPIRAPIM as hazardous substance on November 10, 2014.[4]
See also
References
- ^ "MEPIRAPIM". Cayman Chemical. Retrieved 23 June 2015.
- ^ Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (January 2014). "Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products". Forensic Toxicology. 32 (1): 105–115. doi:10.1007/s11419-013-0217-2. S2CID 32599561.
- ^ Kevin RC, Mirlohi S, Manning JJ, Boyd R, Cairns EA, Ametovski A, et al. (May 2022). "Putative Synthetic Cannabinoids MEPIRAPIM, 5F-BEPIRAPIM (NNL-2), and Their Analogues Are T-Type Calcium Channel (CaV3) Inhibitors". ACS Chemical Neuroscience. 13 (9): 1395–1409. doi:10.1021/acschemneuro.1c00822. PMID 35442021. S2CID 248264685.
- ^ "Cannabinoider föreslås bli klassade som hälsofarlig vara". Retrieved 29 June 2015.
