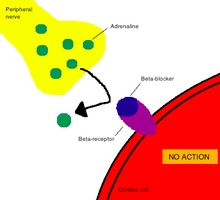
In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect.[2] A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor.[3] Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there.
Drugs that do not bind to receptors produce their corresponding therapeutic effect by simply interacting with chemical or physical properties in the body. Common examples of drugs that work in this way are antacids and laxatives.[2]
In contrast, a mode of action (MoA) describes functional or anatomical changes, at the cellular level, resulting from the exposure of a living organism to a substance.
YouTube Encyclopedic
-
1/5Views:413 875261 18547 64286 40048 227
-
Mechanisms of Hormone Action
-
MECHANISM OF HORMONE ACTION
-
Antacid: Mechanism of Action (Simplified)
-
Digoxin - Mechanism of Action
-
Mechanism of action of Statins
Transcription
The endocrine system is a very important and very involved system within our bodies. It allows our body to communicate over long distances and is a major player in regulating a stable environment or in other words preserving homeostasis. This system employs the use of hormones which are chemicals secreted from glands and enter the bloodstream where they circulate until exerting an effect on a downstream target cell. We're going to be specifically talking about the two types of mechanisms in which hormones exert their effect on target cells. But to start off here's a brief overview what hormones are and how they work. There are three basic group of hormones. 1. Amino acid derivatives. 2. Peptide hormones and 3. Lipid derivatives or steroid hormones. For amino acid derivatives, these are small molecules and they come from amino acids like tyrosine and tryptophan. One hormone derived from tryptophan is melatonin. A hormone involved in our sleep-wake cycles. You can get tryptophan from foods like turkey, chocolate or milk. Peptide hormones are made up of a bunch of amino acids. Some examples would be thyroid-stimulating hormone, oxytocin or prolactin. Lipid derivatives or steroid hormones are considered lipophilic meaning they have an affinity for lipid structures which we'll take a look at shortly. They circulate in the blood bound to carrier proteins so they usually last in the circulation longer than other types of hormones. There are two main mechanisms for which a hormone can affect a target cell. The first is by and non steroid action. This mechanism is employed by an amino acid hormone or a peptide hormone. These hormones cannot freely cross a membrane on a target cell so it first acts by binding a cell surface receptor. There is then an intracellular signaling cascade that occurs in order for the desired effect to take place. There are proteins on the intracellular side of the target cell that are associated with the receptor. Most often this involves what's called a G-protein. This G protein is found next to an enzyme that converts ATP to a molecule called cyclic AMP or cAMP. This is something that makes this mechanism unique. Cyclic AMP is called a second messenger because it signals a cascade of events that eventually change the enzymatic activity in a cell to cause the target cell response. Examples of hormones that use the cyclic AMP second messenger system include ACTH, calcitonin, epinephrine, glucagon, parathyroid hormone and ADH. The second mechanism is the steroid action. Because lipid derivative or steroid hormones are lipophilic they can freely move through the membrane and into the cell. From there they can either bind to a receptor within the cell or they can move freely into the nucleus where the hormone or hormone complex causes a change in gene activity. This then increases transcription and mRNA production which then leads to an increase in protein production within the cell. Examples of hormones that exert effects via steroid hormone mechanisms are testosterone, estrogen, progesterone, aldosterone, and calcitriol.
Importance
Elucidating the mechanism of action of novel drugs and medications is important for several reasons:
- In the case of anti-infective drug development, the information permits anticipation of problems relating to clinical safety. Drugs disrupting the cytoplasmic membrane or electron transport chain, for example, are more likely to cause toxicity problems than those targeting components of the cell wall (peptidoglycan or β-glucans) or 70S ribosome, structures which are absent in human cells.[4][5]
- By knowing the interaction between a certain site of a drug and a receptor, other drugs can be formulated in a way that replicates this interaction, thus producing the same therapeutic effects. Indeed, this method is used to create new drugs.
- It can help identify which patients are most likely to respond to treatment. Because the breast cancer medication trastuzumab is known to target protein HER2, for example, tumors can be screened for the presence of this molecule to determine whether or not the patient will benefit from trastuzumab therapy.[6][7]
- It can enable better dosing because the drug's effects on the target pathway can be monitored in the patient. Statin dosage, for example, is usually determined by measuring the patient's blood cholesterol levels.[6]
- It allows drugs to be combined in such a way that the likelihood of drug resistance emerging is reduced. By knowing what cellular structure an anti-infective or anticancer drug acts upon, it is possible to administer a cocktail that inhibits multiple targets simultaneously, thereby reducing the risk that a single mutation in microbial or tumor DNA will lead to drug resistance and treatment failure.[4][8][9][10]
- It may allow other indications for the drug to be identified. Discovery that sildenafil inhibits phosphodiesterase-5 (PDE-5) proteins, for example, enabled this drug to be repurposed for pulmonary arterial hypertension treatment, since PDE-5 is expressed in pulmonary hypertensive lungs.[11][12]
Determination
Microscopy-based methods

Bioactive compounds induce phenotypic changes in target cells, changes that are observable by microscopy and that can give insight into the mechanism of action of the compound.[13]
With antibacterial agents, the conversion of target cells to spheroplasts can be an indication that peptidoglycan synthesis is being inhibited, and filamentation of target cells can be an indication that PBP3, FtsZ, or DNA synthesis is being inhibited. Other antibacterial agent-induced changes include ovoid cell formation, pseudomulticellular forms, localized swelling, bulge formation, blebbing, and peptidoglycan thickening.[4] In the case of anticancer agents, bleb formation can be an indication that the compound is disrupting the plasma membrane.[14]
A current limitation of this approach is the time required to manually generate and interpret data, but advances in automated microscopy and image analysis software may help resolve this.[4][13]
Direct biochemical methods
Direct biochemical methods include methods in which a protein or a small molecule, such as a drug candidate, is labeled and is traced throughout the body.[15] This proves to be the most direct approach to find target protein that will bind to small targets of interest, such as a basic representation of a drug outline, in order to identify the pharmacophore of the drug. Due to the physical interactions between the labeled molecule and a protein, biochemical methods can be used to determine the toxicity, efficacy, and mechanism of action of the drug.
Computation inference methods
Typically, computation inference methods are primarily used to predict protein targets for small molecule drugs based on computer based pattern recognition.[15] However, this method could also be used for finding new targets for existing or newly developed drugs. By identifying the pharmacophore of the drug molecule, the profiling method of pattern recognition can be carried out where a new target is identified.[15] This provides an insight at a possible mechanism of action since it is known what certain functional components of the drug are responsible for when interacting with a certain area on a protein, thus leading to a therapeutic effect.
Omics based methods
Omics based methods use omics technologies, such as chemoproteomics, reverse genetics and genomics, transcriptomics, and proteomics, to identify the potential targets of the compound of interest.[16] Reverse genetics and genomics approaches, for instance, uses genetic perturbation (e.g. CRISPR-Cas9 or siRNA) in combination with the compound to identify genes whose knockdown or knockout abolishes the pharmacological effect of the compound. On the other hand, transcriptomics and proteomics profiles of the compound can be used to compare with profiles of compounds with known targets. Thanks to computation inference, it is then possible to make hypotheses about the mechanism of action of the compound, which can subsequently be tested.[16]
Drugs with known MOA
There are many drugs in which the mechanism of action is known. One example is aspirin.
Aspirin
The mechanism of action of aspirin involves irreversible inhibition of the enzyme cyclooxygenase;[17] therefore suppressing the production of prostaglandins and thromboxanes, thus reducing pain and inflammation. This mechanism of action is specific to aspirin and is not constant for all nonsteroidal anti-inflammatory drugs (NSAIDs). Rather, aspirin is the only NSAID that irreversibly inhibits COX-1.[18]
Drugs with unknown MOA
Some drug mechanisms of action are still unknown. However, even though the mechanism of action of a certain drug is unknown, the drug still functions; it is just unknown or unclear how the drug interacts with receptors and produces its therapeutic effect.
Mode of action
In some literature articles, the terms "mechanism of action" and "mode of action" are used interchangeably, typically referring to the way in which the drug interacts and produces a medical effect. However, in actuality, a mode of action describes functional or anatomical changes, at the cellular level, resulting from the exposure of a living organism to a substance.[19] This differs from a mechanism of action since it is a more specific term that focuses on the interaction between the drug itself and an enzyme or receptor and its particular form of interaction, whether through inhibition, activation, agonism, or antagonism. Furthermore, the term "mechanism of action" is the main term that is primarily used in pharmacology, whereas "mode of action" will more often appear in the field of microbiology or certain aspects of biology.
See also
References
- ^ Ogrodowczyk, M.; Dettlaff, K.; Jelinska, A. (2016). "Beta-blockers: Current state of knowledge and perspectives". Mini Reviews in Medicinal Chemistry. 16 (1): 40–54. doi:10.2174/1389557515666151016125948. PMID 26471965.
- ^ a b Spratto, G.R.; Woods, A.L. (2010). Delmar Nurse's Drug Handbook. Cengage Learning. ISBN 978-1-4390-5616-5.
- ^ Grant, R.L.; Combs, A.B.; Acosta, D. (2010) "Experimental Models for the Investigation of Toxicological Mechanisms". In McQueen, C.A. Comprehensive Toxicology (2nd ed.). Oxford: Elsevier. p. 204. ISBN 978-0-08-046884-6.
- ^ a b c d e Cushnie, T.P.; O’Driscoll, N.H.; Lamb, A.J. (2016). "Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action". Cellular and Molecular Life Sciences. 73 (23): 4471–4492. doi:10.1007/s00018-016-2302-2. hdl:10059/2129. PMID 27392605. S2CID 2065821. Archived from the original on 2017-10-07. Retrieved 2017-10-07.
- ^ Chang, C.C.; Slavin, M.A.; Chen, S.C. (2017). "New developments and directions in the clinical application of the echinocandins". Archives of Toxicology. 91 (4): 1613–1621. doi:10.1007/s00204-016-1916-3. PMID 28180946. S2CID 31029386.
- ^ a b No authors listed (2010). "Mechanism matters". Nature Medicine. 16 (4): 347. doi:10.1038/nm0410-347. PMID 20376007.
- ^ Joensuu, H. (2017). "Escalating and de-escalating treatment in HER2-positive early breast cancer". Cancer Treatment Reviews. 52: 1–11. doi:10.1016/j.ctrv.2016.11.002. PMID 27866067. Archived from the original on 2019-08-05. Retrieved 2017-10-07.
- ^ Cihlar, T.; Fordyce, M. (2016). "Current status and prospects of HIV treatment". Current Opinion in Virology. 18: 50–56. doi:10.1016/j.coviro.2016.03.004. PMID 27023283.
- ^ Antony, H.A.; Parija, S.C. (2016). "Antimalarial drug resistance: An overview". Tropical Parasitology. 6 (1): 30–41. doi:10.4103/2229-5070.175081. PMC 4778180. PMID 26998432.
- ^ Bozic, I.; Reiter, J.G.; Allen, B.; Antal, T.; Chatterjee, K.; Shah, P.; Moon, Y.S.; Yaqubie, A.; Kelly, N.; Le, D.T.; Lipson, E.J.; Chapman, P.B.; Diaz, L.A.; Vogelstein, B.; Nowak, M.A. (2013). "Evolutionary dynamics of cancer in response to targeted combination therapy". eLife. 2: Article ID e00747. doi:10.7554/eLife.00747. PMC 3691570. PMID 23805382.
- ^ Tari, L.; Vo, N.; Liang, S.; Patel, J.; Baral, C.; Cai, J. (2012). "Identifying novel drug indications through automated reasoning". PLOS ONE. 7 (7): Article e40946. Bibcode:2012PLoSO...740946T. doi:10.1371/journal.pone.0040946. PMC 3402456. PMID 22911721.
- ^ Hayardeny, L. (2014). Why is it important to know the mode of action of drugs? (Conference presentation). New Frontiers in Neuroscience and Methods of Transdisciplinary Education Workshop, Tel Aviv University, Israel: Tel Aviv University. Archived from the original on 18 March 2017. Retrieved 18 March 2017.
- ^ a b Fetz, V.; Prochnow, H.; Brönstrup, M.; Sasse, F. (2016). "Target identification by image analysis" (PDF). Natural Product Reports. 33 (5): 655–667. doi:10.1039/c5np00113g. hdl:10033/621283. PMID 26777141. Archived (PDF) from the original on 2020-06-02. Retrieved 2019-09-26.
- ^ Dubovskii, P.V.; Vassilevski, A.A.; Kozlov, S.A.; Feofanov, A.V.; Grishin, E.V.; Efremov, R.G. (2015). "Latarcins: versatile spider venom peptides". Cellular and Molecular Life Sciences. 72 (23): 4501–4522. doi:10.1007/s00018-015-2016-x. PMID 26286896. S2CID 14177431.
- ^ a b c Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. (2013). "Target identification and mechanism of action in chemical biology and drug discovery". Nature Chemical Biology. 9 (4): 232–240. doi:10.1038/nchembio.1199. ISSN 1552-4450. PMC 5543995. PMID 23508189.
- ^ a b Wecke, T.; Mascher, T. (2011). "Antibiotic research in the age of omics: from expression profiles to interspecies communication". Journal of Antimicrobial Chemotherapy. 66 (12): 2689–2704. doi:10.1093/jac/dkr373. PMID 21930574.
- ^ Tóth, L.; Muszbek, L.; Komaromi, I. (2013). "Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by QM/MM calculations". Journal of Molecular Graphics and Modelling. 40: 99–109. doi:10.1016/j.jmgm.2012.12.013. PMID 23384979.
- ^ Sharma, S.; Sharma, S. C. (1997). "An update on eicosanoids and inhibitors of cyclooxygenase enzyme systems". Indian Journal of Experimental Biology. 35 (10): 1025–1031. ISSN 0019-5189. PMID 9475035.
- ^ "Mechanisms and mode of dioxin action" (PDF). U.S. Environmental Protection Agency. Archived (PDF) from the original on 28 December 2016. Retrieved 11 June 2012.
