| |
| Names | |
|---|---|
| IUPAC name
Molybdenum tetrachloride
| |
| Other names
Molybdenum(IV) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.039 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cl4Mo | |
| Molar mass | 237.752 g/mol |
| Appearance | black solid |
| Melting point | 552 °C (1,026 °F; 825 K) |
| Decomposes | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non flammable |
| Related compounds | |
Related compounds
|
Molybdenum(II) chloride Molybdenum(III) chloride Molybdenum(V) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.
YouTube Encyclopedic
-
1/5Views:1 2253 0511 2989381 002
-
Molybdenum tetrachloride bis(diethyl ether)
-
Molybdenum trichloride tris(tetrahydrofuran)
-
Molybdenum Trisanilide
-
N2 Cleavage Intro Lecture
-
Titanium Trisanilide
Transcription
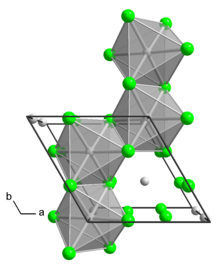
Structure
The α polymorph is a polymer. The β polymorph is a hexamer. In both polymorph, the Mo center is octahedral with two terminal chloride ligands and four doubly bridging ligands.[1] In addition to these two binary phases, a number of adducts are know with the formula MoCl4L2 where L is a Lewis base.
Preparation
α-Molybdenum tetrachloride can be prepared from by dechlorination of molybdenum pentachloride using tetrachloroethene:[2]
- 2 MoCl5 + C2Cl4 → 2 MoCl4 + C2Cl6
Heating α-molybdenum tetrachloride in a sealed container in the presence of molybdenum pentachloride induces conversion to the β polymorph.[2]
Reactions
When heated in an open container, molybdenum tetrachloride evolves chlorine, giving molybdenum trichloride;[2]
- 2 MoCl4 → 2 MoCl3 + Cl2
The acetonitrile complex adduct can be prepared by reduction of the pentachloride with acetonitrile:[3][4]
- 2 MoCl5 + 5 CH3CN → 2 MoCl4(CH3CN)2 + ClCH2CN + HCl
The MeCN ligands can be exchanged with other ligands:
- MoCl4(CH3CN)2 + 2 THF → MoCl4(THF)2 + 2 CH3CN
The pentachloride can be reduced to the ether complex MoCl4(Et2O)2 using tin powder. It is a beige, paramagnetic solid.[5]
References
- ^ Ulrich Müller (1981). "Hexameric Molybdenum Tetrachloride". Angewandte Chemie International Edition in English. 20 (8): 692. doi:10.1002/anie.198106921.
- ^ a b c McCann III, E. L.; Brown, T. M. (1970). "Molybdenum(IV) Chloride". Inorganic Syntheses. Inorganic Syntheses. Vol. 12. p. 181. doi:10.1002/9780470132432.ch31. ISBN 9780470132432.
- ^ Broderick, Erin M.; Browne, Samuel C.; Johnson, Marc J. A. (2014). "Dimolybdenum and Ditungsten Hexa(Alkoxides)". Inorganic Syntheses: Volume 36. Inorganic Syntheses. Vol. 36. pp. 95–102. doi:10.1002/9781118744994.ch18. ISBN 9781118744994.
- ^ Dilworth, Jonathan R.; Richards, Raymond L. (1990). "The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes". Inorganic Syntheses. Inorganic Syntheses. Vol. 28. p. 33. doi:10.1002/9780470132593.ch7. ISBN 9780470132593.
- ^ Maria, Sébastien; Poli, Rinaldo (2014). "Ether Complexes of Molybdenum(III) and Molybdenum(IV) chlorides". Inorganic Syntheses: Volume 36. Inorganic Syntheses. Vol. 36. pp. 15–18. doi:10.1002/9781118744994.ch03. ISBN 9781118744994.

