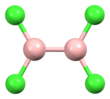
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Diboron tetrachloride | |||
| Systematic IUPAC name
Tetrachlorodiborane(4) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| B2Cl4 | |||
| Molar mass | 163.42 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 1.5 g/cm3 (0 °C) | ||
| Melting point | −92.6 °C (−134.7 °F; 180.6 K) | ||
| Boiling point | 65.5 °C (149.9 °F; 338.6 K) | ||
| Thermochemistry | |||
Heat capacity (C)
|
137.7 J/mol K | ||
Std molar
entropy (S⦵298) |
232.3 J/mol K | ||
Std enthalpy of
formation (ΔfH⦵298) |
-523 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
-468.8 kJ/mol | ||
| Related compounds | |||
Related compounds
|
Diboron tetrafluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Diboron tetrachloride is a chemical compound with the formula B2Cl4. It is a colorless liquid.[1]
Synthesis
The modern synthesis involves dechlorination of boron trichloride using copper.[2]
It can also be formed by the electrical discharge procedure of boron trichloride at low temperatures:[1][3]
- BCl3 → BCl2 + Cl
- Cl + Hg (electrode) → HgCl or HgCl2
- 2 BCl2 → B2Cl4
Reactions
The compound is used as a reagent for the synthesis of organoboron compounds. For instance, diboron tetrachloride reacts with ethylene:[4]
- CH2=CH2 + B2Cl4 → Cl2B–CH2–CH2–BCl2
The compound absorbs hydrogen quickly at room temperature:[3]
- 3 B2Cl4 + 3 H2 → B2H6 + 4 BCl3
References
- ^ a b P. L. Timms (1972). Low Temperature Condensation. Academic Press. p. 143. ISBN 0-12-023614-1.
{{cite book}}:|work=ignored (help) - ^ Timms, Peter L. (1979). "Tetrachlorodiborane(4) (Diboron Tetrachloride)". Inorganic Syntheses. Vol. 19. pp. 74–78. doi:10.1002/9780470132500.ch14. ISBN 9780470132500.
- ^ a b Urry, Grant; Wartik, Thomas; Moore, R. E.; Schlesinger, H. I. (1954). "The Preparation and Some of the Properties of Diboron Tetrachloride, B2Cl4". Journal of the American Chemical Society. 76 (21): 5293–5298. doi:10.1021/ja01650a010. ISSN 0002-7863.
- ^ Urry, Grant; Kerrigan, James; Parsons, Theran D.; Schlesinger, H. I. (1954). "Diboron Tetrachloride, B2Cl4, as a Reagent for the Synthesis of Organo-boron Compounds. I. The Reaction of Diboron Tetrachloride with Ethylene". Journal of the American Chemical Society. 76 (21): 5299–5301. doi:10.1021/ja01650a011. ISSN 0002-7863.