| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chloro perchlorate[1]
| |||
| Systematic IUPAC name
Chloro perchlorate[1] | |||
| Other names
Chlorine(I,VII) oxide
Dichlorine tetroxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
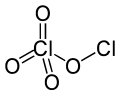
| Cl2O4 | |||
| Molar mass | 134.90 g·mol−1 | ||
| Appearance | Pale green liquid | ||
| Density | 1.81 g·cm−3 | ||
| Melting point | −117 °C (−179 °F; 156 K) | ||
| Boiling point | 20 °C (68 °F; 293 K) (decomposes) | ||
| Reacts | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
oxidizer | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:[2][3][4]
- 2ClO2 → ClOClO3
Chlorine perchlorate can also be made by the following reaction at −45 °C.
- CsClO4 + ClOSO2F → Cs(SO3)F + ClOClO3
YouTube Encyclopedic
-
1/5Views:155 61730 955195 5383 250 82114 545
-
Ammonium perchlorate: NH4ClO4. Rocket fuel from construction foam!
-
NOClO4: Nitrosyl perchlorate
-
Make Potassium Chlorate by Electrolysis - The Basic Guide
-
Don't Mix These Chemicals! What Happens When You Mix Brake Fluid and Chlorine? TKOR Shows You!
-
Perchlorate production via electrolysis, with PbO2 anode
Transcription
Properties
Chlorine perchlorate is a pale greenish liquid. It is less stable than ClO2 (chlorine dioxide)[citation needed] and decomposes at room temperature to give O2 (oxygen), Cl2 (chlorine) and Cl2O6 (dichlorine hexoxide):
- 2ClOClO3 → O2 + Cl2 + Cl2O6
Chlorine perchlorate reacts with metal chlorides to form chlorine and the corresponding anhydrous perchlorate:
- CrO2Cl2 + 2ClOClO3 → 2Cl2 + CrO2(ClO4)2
- TiCl4 + 4ClOClO3 → 4Cl2 + Ti(ClO4)4
- 2AgCl + 2 ClOClO3 → 2AgClO4 + Cl2
Reactions
| Reactant | Conditions | Products |
|---|---|---|
| — | Heat | dichlorine hexoxide (80%), chlorine dioxide, chlorine, oxygen |
| — | Ultraviolet light | dichlorine heptoxide, chlorine, oxygen[4] |
| caesium iodide | −45 °C | Cs[I(OClO3)4][note 1] |
| ClOSO2F or ClF | — | MClO4(M = Cs or NO2)[note 2] |
| bromine | −45 °C | bromine perchlorate (BrOClO3)[note 2] |
| iodine(0.33 mol) | −50 °C | I(OClO3)3[note 3] |
Notes
- ^ Cs[I(OClO3)4] is a pale yellow salt which is stable at room temperature. It has a square IO4 unit.
- ^ a b MClO4 (M = Cs or NO2) reacts with BrOSO2F at −20 °C and produces bromine perchlorate (BrOClO3). Bromine perchlorate then reacts with hydrogen bromide (HBr) at −70 °C and produces elemental bromine (Br2) and perchloric acid (HClO4).
- ^ The last[5] attempt to form iodine monoperchlorate (IOClO3) occurred in 1972,[6] and even at low temperatures yielded instead the triperchlorate. On warming, the latter then decomposes to iodate.
References
- ^ a b "Chloro Perchlorate - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ A. J. Schell-Sorokin; D. S. Bethune; J. R. Lankard; M. M. T. Loy; P. P. Sorokin (1982). "Chlorine perchlorate a major photolysis product of chlorine dioxide". J. Phys. Chem. 86 (24): 4653–4655. doi:10.1021/j100221a001.
- ^ M. I. Lopez; J. E. Sicre (1988). "Ultraviolet spectrum of chlorine perchlorate". J. Phys. Chem. 92 (2): 563–564. doi:10.1021/j100313a062.
- ^ a b Rao, Balaji; Anderson, Todd A.; Redder, Aaron; Jackson, W. Andrew (2010-04-15). "Perchlorate Formation by Ozone Oxidation of Aqueous Chlorine/Oxy-Chlorine Species: Role of ClxOy Radicals". Environmental Science & Technology. 44 (8): 2961–2967. Bibcode:2010EnST...44.2961R. doi:10.1021/es903065f. ISSN 0013-936X. PMID 20345093.
- ^ Zefirov, N. S.; Zedankin, V. V.; Koz'min, A. S. (1988). "The synthesis and properties of covalent organic perchlorates". Russian Chemical Reviews. Turpion. 57 (11): 1047. doi:10.1070/RC1988v057n11ABEH003410. Translated from Uspekhi Khimii volume 57 (1988), pp. 1815-1839.
- ^ Christe, Karl O.; Schack, Carl J. (1972) [20 September 1971]. "Iodine tris(perch1orate) and cesium tetrakis(perchlorato)iodate(III)". Inorganic Chemistry. 11 (7): 1684. doi:10.1021/ic50113a047.