| |
| Names | |
|---|---|
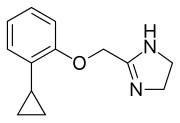
| Preferred IUPAC name
2-[(2-Cyclopropylphenoxy)methyl]-4,5-dihydro-1H-imidazole | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Cirazoline |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H16N2O | |
| Molar mass | 216.284 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cirazoline is a full agonist at the α1A adrenergic receptor, a partial agonist at both the α1B and α1D adrenergic receptors,[1] and a nonselective antagonist to the α2 adrenergic receptor.[2] It is believed that this combination of properties could make cirazoline an effective vasoconstricting agent.[2]
Cirazoline has also been shown to decrease food intake in rats, purportedly through activation of α1 adrenoceptors in the paraventricular nucleus in the hypothalamus of the brain.[3] Administration of cirazoline also seemed to present impairment in the spatial memory of monkeys through the activation of the same receptors that showed decreased food intake in rats.[4][5] However, in preliminary studies, through stimulation of α2 adrenoceptors, working memory is comparatively improved.[4]
References
- ^ Horie, K; Obika, K; Foglar, R. (1995). "Selectivity of the imidazoline α-adrenoceptor agonists (oxymetazoline and cirazoline) for human cloned α1-adrenoceptor subtypes". British Journal of Pharmacology. 116 (1): 1611–8. doi:10.1111/j.1476-5381.1995.tb16381.x. PMC 1908909. PMID 8564227.
- ^ a b Ruffolo, R. R. Jr.; Waddell, J. E. (1982). "Receptor interactions of imidazolines. IX. Cirazoline is an α1 adrenergic agonist and an α2 adrenergic antagonist". Journal of Pharmacology and Experimental Therapeutics. 222 (1): 29–36. PMID 6123592.
- ^ Davies, B. T.; Wellman, P. J. (1992). "Effects on ingestive behavior in rats of the α1-adrenoceptor agonist cirazoline". European Journal of Pharmacology. 210 (1): 11–16. doi:10.1016/0014-2999(92)90645-K. PMID 1350985.
- ^ a b Arnsten, A.F.T.; Jentsch, J.D. (September 1997). "The Alpha-1 Adrenergic Agonist, Cirazoline, Impairs Spatial Working Memory Performance in Aged Monkeys". Pharmacology Biochemistry and Behavior. 58 (1): 55–59. doi:10.1016/s0091-3057(96)00477-7. ISSN 0091-3057. PMID 9264070. S2CID 20663570.
- ^ Imbery, Irdmusa, Speidell, Streer, Griffin, Ted E., Mitra S., Andrew P., Mark S., John D. (15 December 2007). "The effects of Cirazoline, an alpha-1 adrenoreceptor agonist, on the firing rates of thermally classified anterior hypothalamic neurons in rat brain slices". Brain Research. 1193: 93–101. doi:10.1016/j.brainres.2007.12.016. PMC 2268753. PMID 18184607.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
