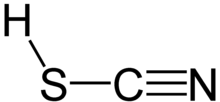
| |
 | |
| Names | |
|---|---|
| IUPAC name
Thiocyanic acid[4]
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.672 |
| EC Number |
|
| 25178 | |
| KEGG | |
| MeSH | thiocyanic+acid |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HSCN | |
| Molar mass | 59.09 g·mol−1 |
| Appearance | |
| Odor | Pungent |
| Density | 2.04 g/cm3 |
| Melting point | |
| Miscible | |
| Solubility | Soluble in ethanol, diethyl ether |
| log P | 0.429 |
| Vapor pressure | 4.73 mmHg (631 Pa)[7] |
| Acidity (pKa) | 0.926 |
| Basicity (pKb) | 13.071 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H332, H412 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thiocyanic acid is a chemical compound with the formula HSCN and structure H−S−C≡N, which exists as a tautomer with isothiocyanic acid (H−N=C=S).[8] The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase.[9]
It is a moderately strong acid,[10] with a pKa of 1.1 at 20 °C and extrapolated to zero ionic strength.[11]
One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen. Thiocyanic acid has been observed spectroscopically.[12]
The salts and esters of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion ([SCN]−) and a suitable cation (e.g., potassium thiocyanate, KSCN). The esters of thiocyanic acid have the general structure R−S−C≡N, where R stands for an organyl group.
Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C have been reported.[13] HNCS acceptor properties are discussed in the ECW model. The salts are composed of the thiocyanate ion ([SCN]−) and a suitable cation (e.g., ammonium thiocyanate, [NH4]+[SCN]−). Isothiocyanic acid forms isothiocyanates R−N=C=S, where R stands for an organyl group.
YouTube Encyclopedic
-
1/5Views:5563 1727 3221 251654
-
Thiocyanic acid
-
Perthiocyanic Acid - Yellow Chem GOOD!
-
HSCN Lewis Structure: Thiocyanic Acid
-
Thiocyanate
-
Thiocyanate Meaning
Transcription
References
- ^ Merck Index, 11th Edition, 9257.
- ^ a b "Thiocyanic acid". The Merck Index. Royal Society of Chemistry.
- ^ von Richter, Victor (1922). Organic Chemistry or Chemistry of the Carbon Compounds. Vol. 1. Translated by Spielmann, Percy E. Philadelphia: P. Blakiston's Son & Co. p. 466.
- ^ "Thiocyanic acid" entry in PubChem (database).
- ^ a b ILO and WHO staff. "Thiocyanic acid" safety card. European Commission
- ^ Birckenbach, Lothar (1942). Forschungen und Fortschritte. 18: 232–3
{{cite journal}}: Missing or empty|title=(help). As cited in CAS Common Chemistry. - ^ Brown, Jay A. (ed.; 2024), "Thiocyanic Acid" in Haz-Map (database). Engineered IT.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Beard, C. I.; Dailey, B. P. (1950). "The Structure and Dipole Moment of Isothiocyanic Acid" (PDF). The Journal of Chemical Physics. 18 (11): 1437. Bibcode:1950JChPh..18.1437B. doi:10.1063/1.1747507. hdl:1721.1/4934.
- ^ Munegumi, Toratane (23 January 2013). "Where is the Border Line between Strong Acids and Weak Acids?". World Journal of Chemical Education. 1 (1): 12–16.
- ^ Martell, A. E.; Smith, R. M.; Motelaitis, R. J. (2001). NIST Database 46. Gaithersburg, MD: National Institute of Standards and Technology.
- ^ Wierzejewska, M.; Mielke, Z. (2001). "Photolysis of Isothiocyanic Acid HNCS in Low-Temperature Matrices. Infrared Detection of HSCN and HSNC Isomers". Chemical Physics Letters. 349 (3–4): 227–234. Bibcode:2001CPL...349..227W. doi:10.1016/S0009-2614(01)01180-0.
- ^ . Barakat, T. M.; Nelson, J.; Nelson, S. M.; Pullin, A. D. E. (1969.) “Spectra and hydrogen-bonding of characteristics of thiocyanic acid. Part 4.—Association with weak proton acceptors”. Trans. Faraday Soc., 1969,65, 41-51


