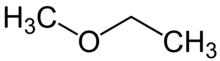
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methoxyethane[1] | |
| Other names
ethyl methyl ether
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.128.000 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H8O | |
| Molar mass | 60.096 g·mol−1 |
| Appearance | Colorless gas[2] |
| Density | 0.7251 g cm−3 (at 0 °C)[2] |
| Melting point | −113 °C (−171 °F; 160 K) |
| Boiling point | 7.4 °C (45.3 °F; 280.5 K) |
Refractive index (nD)
|
1.3420 (at 4 °C)[2] |
| Viscosity | 0.224 cP at 25 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely Flammable (F+), Liquefied gas |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related Ethers
|
Dimethyl ether Diethyl ether Methoxypropane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methoxyethane, also known as ethyl methyl ether, is a colorless gaseous ether with the formula CH3OCH2CH3. Unlike the related dimethyl ether and diethyl ether, which are widely used and studied, this mixed alkyl ether has no current applications. It is a structural isomer of isopropyl alcohol. Its utility as an anesthetic[3] and solvent[4] have been investigated.
YouTube Encyclopedic
-
1/5Views:90 231238 960420 13117 59823 691
-
Naming Ethers using IUPAC Nomenclature and Common Names in Organic Chemistry
-
Ether naming and introduction | Organic chemistry | Khan Academy
-
Hydrogen Bonding and Common Mistakes
-
Is CH3OCH3 Polar or Nonpolar? - Dimethyl Ether
-
C2H6O Lewis Structure: How to Draw the Lewis Structure for C2H6O
Transcription
Leah here from www.Leah4Sci.com and in this video, I will show you how to name ethers. An ether is a molecule typically written out as R bound to O bound to R prime where R simply represents the rest of the molecule or some carbon chain and R prime can either be the same R group or a different R group. For example, we can have an ether created by connecting two methyl groups to an oxygen or creating both a methyl and an ethyl group to an oxygen. There are two common ways to name ethers. The IUPAC rules follow the same rules we�ve been using for naming organic compounds. You start with your longest carbon chain as your parent chain. On the left we have methyl giving me a first name of �meth�. Only single bonds gives me a last name of �ane�. The shorter carbon chain attached to the oxygen becomes an alkoxy substituent where you simply take the prefix for the carbon number and add the word �oxy�. In this case we will have methoxy. Since carbon 1 is understood, we ignore the number giving this a final name of methoxymethane. Same thing applies to my second ether. In this case my longest carbon chain has two carbons, giving me a first name of �eth�. Only single bonds gives me a last name of �ane�. A one-carbon substituent on the oxygen gives me a 1-methoxy. Once again carbon 1 is understood and can be ignored for a final name of methoxyethane. The common name for an ether involves naming the two substituents that are attached to the oxygen followed by the word �ether�. If the two groups are different, you can arrange them in alphabetical order. Looking back at the first one, I have a methyl group on the left. It gives me methyl. A methyl group on the right would give me another methyl but since it�s two of the same, I will use dimethyl followed by the word �ether�. On the right we have both a methyl and an ethyl. Arranged in alphabetical order gives me ethyl for the longer chain, methyl for the shorter chain, followed by ether. When the ether is part of a larger molecule, you still follow the same rules for naming organic compounds. In this example, we start by identifying the parent chain and numbering from the side that gives the ether the lowest number. In this case, I number from the left and get a total of four carbons for a first name of �but�. Having only single bonds gives me a last name of �ane�. I have a two-carbon ether substituent coming off of carbon 2 for a prefix of �2-ethoxy�. Putting the name together, I get 2-ethoxybutane. If you�re given an ether with cyclic substituents, don�t let the apparent complexity fool you. We will start by identifying the parent chain and follow the regular naming. In this case, six carbons gives me a first name of �hex.� Single bonds gives me a last name of �ane� and the fact that it�s a ring gives me a cyclo. Since my oxy substituent is five carbons in a ring, I have the prefix cyclopentoxy. The number 1 is understood given that I have nothing else on the molecule, giving me a final name of cyclopentoxycyclohexane. When you have more than one ether on a molecule, you name it the same way. Include a number for the carbon holding each ether and include the word �di� to show that you have 2. I start by identifying and highlighting my parent chain. I have a total of five carbons on the symmetrical molecule allowing me to number from either direction. Five carbons gives me a first name of �pent�. Only single bonds gives me a last name of �ane�. Both ethers have two carbons appearing one on carbon 1, the other on carbon 5 for a prefix of �1, 5-diethoxy�. This gives me a final name of 1, 5-diethoxypentane. When you have an alcohol and ether on the same molecule, the OH group takes priority. In this case, the molecule is numbered so that OH rather than OCH3 gets the lower number which means I have to number from the right. Four carbons gives me a first name of �but�. Only single bonds gives me a last name of �ane�. Since the alcohol is higher priority, I have a functional group of �2-ol�. The ether is my substituent giving me 3-methoxy. Pulling 2 in front of the first name means that O will directly follow E and so I have to drop the letter E for a final name of 3-methoxy-2-butanol. When you have two carbons and an oxygen in a ring, this is called an epoxide or oxirane and will come up a lot in organic chemistry reactions. Therefore in the next video, we will look at how to name these specifically in detail.
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 703. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ a b c Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida, USA: CRC Press. p. 3-248. ISBN 978-1-43982077-3.
- ^ Bovill, J. G. (2008). "Inhalation Anaesthesia; From Diethyl Ether to Xenon". Modern Anesthetics. Handbook of Experimental Pharmacology. Vol. 182. Springer. pp. 121–142. doi:10.1007/978-3-540-74806-9_6. ISBN 978-3-540-72813-9. PMID 18175089.
- ^ Campion, Christopher L.; Li, Wentao; Lucht, Brett L. (2005). "Thermal Decomposition of LiPF[sub 6]-Based Electrolytes for Lithium-Ion Batteries". Journal of the Electrochemical Society. 152 (12): A2327. doi:10.1149/1.2083267.

