 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H13N3O |
| Molar mass | 203.245 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
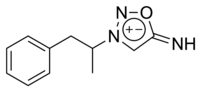
Feprosidnine (Sydnophen) is a stimulant drug which was developed in the USSR in the 1970s.[1][2] It is structurally related to another Russian drug mesocarb but unlike mesocarb, was withdrawn earlier from production. In comparison with mesocarb it has own antidepressant activity, which makes it useful in treating depressions. Indications of feprosidnine included apathic, asthenic depressions, fatigue, apathic syndrome, narcolepsy and other similar conditions. Therapeutic range of doses: 10-50mg a day. Sydnophen has multiple mechanisms of action, the relative importance of which has not been clearly established. Effects on the body include reversible monoamine oxidase inhibition, cholinergic,[3] adrenergic,[4] opioid[5] and nitric oxide donating[6] actions, all of which may contribute to its pharmacological effects to some extent.
Chemistry
Feprosidnine is a mesoionic sydnone imine.
A similar agent is described in the Amfetaminil article.
Synthesis

Base catalyzed reaction between acetone cyanohydrin and 40% formaldehyde solution gives glycolonitrile (1) & some acetone by-product. The amphetamine [300-62-9] (2) is then added and this is allowed to react overnight to give N-(1-phenyl-2-propylamine)-acetonitrile (3). Nitrosylation of the amino group by in situ creation of nitrous acid from hydrochloric acid and sodium nitrite results in a 51.5% yield of feprosidnine based on the initial weight of the amine (4).
References
- ^ Veksler IG, Riabukha VN, Balitskiĭ KP, Al'tshuler RA, Mashkovskiĭ MD (1980). "[Immunostimulating and antitumor action of psychotropic preparations of the sydnonimine series]". Farmakologiia I Toksikologiia (in Russian). 43 (3): 349–52. PMID 7449977.
- ^ Koniaeva EI, Beketov AI (1987). "[Effect of caffeine and sidnofen on the blood supply of the brain, kidneys and hindlimbs during antiorthostatism]". Farmakologiia I Toksikologiia (in Russian). 50 (3): 39–42. PMID 3609274.
- ^ Samonina GE, Mandriko EV (April 1989). "[Peripheral cholinolytic action--one of the effects of sidnofen]". Biulleten' Eksperimental'noi Biologii I Meditsiny (in Russian). 107 (4): 449–51. PMID 2720163.
- ^ Babskaia NE (1992). "[Sidnofen-dependent pre- and postsynaptic activation of peripheral adrenergic transmission]". Eksperimental'naia i Klinicheskaia Farmakologiia (in Russian). 55 (5): 21–5. PMID 1339046.
- ^ D'iakonova TL, Samonina GE (1994). "[The naloxone-dependent effects of the psychostimulant sidnofen: a study on identified neurons of the snail]". Zhurnal Vysshei Nervnoi Deiatelnosti Imeni I P Pavlova (in Russian). 44 (4–5): 786–95. PMID 7810220.
- ^ Arzamastsev AP, Severina IS, Grigor'ev NB, Granik VG (2003). "[Exogenous donors of nitric oxide and inhibitors of NO-synthase (chemical aspects)]". Vestnik Rossiiskoi Akademii Meditsinskikh Nauk (in Russian) (12): 88–95. PMID 14724985.
- ^ Mashkovsky Mikhail Davydovich & Yashunsky Vladimir Genrikhovic; GB1242743 (1971 to Vni Khim Farmatsevtichesky I I [RU]).
