 | |
| Clinical data | |
|---|---|
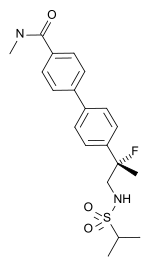
| Other names | LY-503430; (R)-4'-[1-fluoro-1-methyl-2-(propane-2-sulfonylamino)-ethyl]-biphenyl-4-carboxylic acid methylamide |
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H25FN2O3S |
| Molar mass | 392.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
LY-503430 is an AMPA receptor positive allosteric modulator developed by Eli Lilly.[1]
LY-503430 produces both nootropic and neuroprotective effects, reducing brain damage caused by 6-hydroxydopamine or MPTP and also increasing levels of the neurotrophic factor BDNF in the brain, particularly in the substantia nigra, hippocampus, and striatum.[2][3] It is orally active and the main application it is currently being developed for is treatment of Parkinson's disease, although it has also been proposed to be useful in the treatment of Alzheimer's disease, depression, and schizophrenia.[4][5]
YouTube Encyclopedic
-
1/2Views:7 644526 063
-
8:00 PM - All SSC Exams 2020-21 | General Science by Aman Sharma | Science Live Test (100 Questions)
-
Matematika Kelas 9 - Fungsi Kuadrat (2) - Grafik Fungsi Kuadrat, Sumbu Simetri, Titik Potong
Transcription
See also
References
- ^ O'Neill MJ, Murray TK, Clay MP, Lindstrom T, Yang CR, Nisenbaum ES (2005). "LY503430: pharmacology, pharmacokinetics, and effects in rodent models of Parkinson's disease". CNS Drug Reviews. 11 (1): 77–96. doi:10.1111/j.1527-3458.2005.tb00037.x. PMC 6741716. PMID 15867954.
- ^ Murray TK, Whalley K, Robinson CS, Ward MA, Hicks CA, Lodge D, et al. (August 2003). "LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease". The Journal of Pharmacology and Experimental Therapeutics. 306 (2): 752–62. doi:10.1124/jpet.103.049445. PMID 12730350. S2CID 86751458.
- ^ Ryder JW, Falcone JF, Manro JR, Svensson KA, Merchant KM (October 2006). "Pharmacological characterization of cGMP regulation by the biarylpropylsulfonamide class of positive, allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors". The Journal of Pharmacology and Experimental Therapeutics. 319 (1): 293–8. doi:10.1124/jpet.106.105734. PMID 16803862. S2CID 11732324.
- ^ O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES (June 2004). "AMPA receptor potentiators for the treatment of CNS disorders". Current Drug Targets. CNS and Neurological Disorders. 3 (3): 181–94. doi:10.2174/1568007043337508. PMID 15180479.
- ^ O'Neill MJ, Witkin JM (May 2007). "AMPA receptor potentiators: application for depression and Parkinson's disease". Current Drug Targets. 8 (5): 603–20. doi:10.2174/138945007780618517. PMID 17504104.
