| |
| Names | |
|---|---|
| Other names
potassium heptanitrosyltri-μ3-thiotetraferrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Fe4KN7O7S3 | |
| Molar mass | 568.70 g·mol−1 |
| Appearance | Black solid |
| Melting point | 198 to 200 °C (388 to 392 °F; 471 to 473 K) |
| Related compounds | |
Related
|
Roussin's Red Salt |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Roussin's black salt is a chemical compound with the formula KFe4S3(NO)7.[1] It consists of the potassium salt of the [Fe4S3(NO)7]− anion, metal nitrosyl compound. First described by Zacharie Roussin in 1858,[2] it is one of the first synthetic iron-sulfur clusters along with the red salt also bearing his name.
YouTube Encyclopedic
-
1/3Views:864314933
-
CHEMICAL PRESERVATIVES- FOOD SAFETY OFFICER CRASH COURSE DAY-12. #BIOLOGYTUTOR
-
PART 10: SYNTHESIS, PROPERTIES & IR SPECTRA OF METAL NITROSYLS FOR CSIR NET/GATE/JAM
-
Organic Chemistry in Academia Using Real-Time In Situ FTIR
Transcription
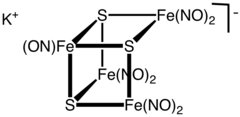
Structure
The cluster anion has the geometry of an incomplete cubane-type cluster with C3v symmetry. The dark colour of the complex is attributed to a number of charge-transfer interactions.[3]

Synthesis
Roussin’s black salt is produced by the reaction of nitrous acid, potassium hydroxide, potassium sulfide, and iron(II) sulfate in aqueous solution.[4] It can also be formed by the conversion of Roussin's red salt in mildly acidic conditions. This reaction is reversible and Roussin’s red salt is reformed upon alkalization of the reaction solution.
Uses
Roussin’s black salt is a nitric oxide donor.[5] Also, Roussin’s Black Salt exhibits antibacterial activity in some food processing applications.[6]
See also
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1094. ISBN 978-0-08-037941-8.
- ^ Butler, Anthony R. (July 1982). "The chemist Z. Roussin (1827-94)". Journal of Chemical Education. 59 (7): 549. Bibcode:1982JChEd..59..549B. doi:10.1021/ed059p549.
- ^ Jaworska, Maria; Stasicka, Zofia (March 2006). "Structure and UV–vis spectroscopy of roussin black salt [Fe4S3(NO)7]−". Journal of Molecular Structure. 785 (1–3): 68–75. Bibcode:2006JMoSt.785...68J. doi:10.1016/j.molstruc.2005.09.030.
- ^ Marchlewski, L.; Sachs, J. (1892). "Studien über ROUSINS Salz". Zeitschrift für anorganische Chemie. 2 (1): 175–181. doi:10.1002/zaac.18920020117.
- ^ Janczyk, Agnieszka; Wolnicka-Glubisz, Agnieszka; Chmura, Antonina; Elas, Martyna; Matuszak, Zenon; Stochel, Grazyna; Urbanska, Krystyna (February 2004). "NO-dependent phototoxicity of Roussin's black salt against cancer cells". Nitric Oxide. 10 (1): 42–50. doi:10.1016/j.niox.2004.01.009. PMID 15050534.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1094–1095. ISBN 978-0-08-037941-8.
