Non-marketed appetite suppressant drug
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
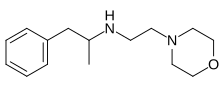
| Formula | C15H24N2O |
| Molar mass | 248.370 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Morforex (INN; Bo 637), also referable to as N-morpholinoethylamphetamine, is an anorectic which was never marketed.[1][2]
It produces amphetamine as an active metabolite.[3]
Synthesis

Amphetamine is reacted with N-Chloroethylmorpholine [3240-94-6] in the presence of IPA solvent.
See also
References
- ^ Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1377–. ISBN 978-0-412-46630-4.
- ^ World Health Organization (2000). International Nonproprietary Names (INN) for Pharmaceutical Substances. World Health Organization. ISBN 978-0-11-986227-0.
- ^ Goodwin BL (10 November 2004). Handbook of Biotransformations of Aromatic Compounds. CRC Press. pp. 13–. ISBN 978-0-203-64196-5.
- ^ FR 2199M, "N-morpholino-1 n-phénylisopropylamino-2 éthane [N-morpholino-1 N-phenylisopropylamino-2 ethane]", published 1963-12-09, assigned to Brevets Pharmaceutiques et Cosmetologiques
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
