| Protein-serine/threonine kinases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.11.- | ||||||||
| CAS no. | 9026-43-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||

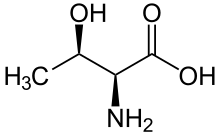
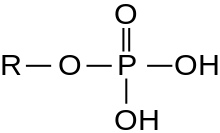

A serine/threonine protein kinase (EC 2.7.11.-) is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK).[2]
In enzymology, the term serine/threonine protein kinase describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important post-translational modification.[3][4][5][6][7][8][9]
The chemical reaction performed by these enzymes can be written as
- ATP + a protein ADP + a phosphoprotein
Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein.
The systematic name of this enzyme class is ATP:protein phosphotransferase (non-specific).
YouTube Encyclopedic
-
1/5Views:190 024107 03819 619273 54111 155
-
Enzyme Linked Receptors | Nervous system physiology | NCLEX-RN | Khan Academy
-
Cell Signaling- Tyrosine Kinase receptors
-
Susan Taylor (UCSD) Part 2: Architecture of a Protein Kinase
-
Receptor Tyrosine Kinase
-
Akt pathway
Transcription
Voiceover: In this video we're gonna learn about enzyme-linked receptors. Like all cell membrane receptors enzyme-linked receptors receives signal from the environment and they instruct the cell to do certain things. Like most, enzyme-linked receptors are transmembrane proteins but they are unique because in addition to receiving signals from chemical messengers they also function as enzymes. Binding of a signaling molecule activates the receptor's enzymatic activity. Enzymes are a substance in our body that act as a catalyst which can speed up particular biochemical reactions. Enzyme-linked receptors are also called catalytic receptors. Over here I've predrawn a picture of our cell membrane. This is our phospholipid by layer. Up top I'm gonna say this is our extra cellular environment and below is our intracellular environment. This is inside our cell where our cytosol and all of our organelles are located. Let's talk a little bit about the structure of enzyme-linked receptors. The general structure of enzyme-linked receptors are shaped like this. Up top here you can see there is a shape which can bind a ligand. This over here is our ligand-binding domain. This is our extracellular portion. Down here, this half of the protein on the intracellular side is our enzymatic domain. It's our functional domain. This is the part of the enzyme-linked receptor that can act as an enzyme. When we have a ligand up here and what it binds in. The extracellular side can bind a ligand which will cause the intracellular side to act as an enzyme. Though there are many different types of enzyme-linked receptors, the most widely recognized and most common enzyme-linked receptors are called receptor tyrosine kinases. They're particularly important because they regulate cell growth, differentiation and survival. They can bind and respond to ligands such as growth factors. These are also called RTKs for short. The structure and function of RTKs aren't really a mystery. It really is writing the name. Part of the reason why receptor tyrosine kinases are unique is because they have tyrosine. If you go ahead and draw out a receptor like this. They're unique because tyrosine is on the intracellular enzymatic section. We can have tyrosine like this. Now that we've addressed the tyrosine portion of the name what do you suppose a kinase means? A kinase is a general term for something that has the ability to transfer phosphorus molecules. Usually from a high energy substance like ATP. Receptor tyrosine kinases have the ability to transfer phosphorus from ATP to intracellular proteins which activates them. That's the enzymatic function of receptor tyrosine kinases to transfer these phosphorus molecules. These proteins which are now phosphorylated can carry out a message through signal transduction. Now let's talk a little bit in more detail about this particular process. We'll talk about why in a second but receptor tyrosine kinases occur in pairs. If you can find one receptor tyrosine kinase you'll find another one that's fairly nearby. Down here we have our tyrosine. Out here we have our extracellular signal. Now let's say that this signal is now binding into that ligand-binding site. What's unique about receptor tyrosine kinase is that these two pairs are gonna come together and act together. Let's go ahead and draw these two pairs close together like this. At this point, our ligand is bound. We have our tyrosine on the bottom here. When this signaling molecule binds to an RTK they cause neighboring RTKs to associate with each other forming what we call a cross-linked dimer. This new thing that's formed when these two come together is a cross-linked dimer. RTKs need to act in pairs. Now the reason why is because cross-linking activates the tyrosine kinase activity in these RTKs through phosphorylation. Now these tyrosine are active and they can start getting phosphorus's. Each RTK in the dimer phosphorylates the tyrosines on the other RTK. There aren't always two tyrosines. There usually are multiple ones. For the sake of clarity I've only drawn in two though. This process of one phosphorylating the other is called cross-phosphorylation. If we have ATP inside the cell these tyrosines will cause it to become ADP with a phosphate group. This tyrosine molecule now that we have our cross-linked dimer is going to go ahead and pick up this free floating phosphate group. Now at a certain point each one of these, each one of the tyrosines are gonna get a phosphate group from ATP. Again, the reason why they need to act in pair is because one receptor tyrosine kinase will phosphorylate the other one. Once cross-phosphorylated, the intracellular cytoplasmic section so the enzymatic section of these RTKs serve as docking platforms for different intracellular proteins involved in signal transduction. Once we have these phosphorus's on the tyrosine different proteins can come by and attach themselves to them. For example, we could have one type of protein and we could also have another type of protein. They don't have to be the same one. Now the only thing that these proteins really need to have to dock with the phosphorus is a special domain specifically called SH2. This can bind to these phosphorylated tyrosines. Again, multiple different SH2-containing proteins can bind at the same time to any of these phosphorus. We've only drawn proteins on this one side but the same or different proteins can also bind on the other side. This allows activation of multiple different intracellular signaling pathways at the same time. Now after that, the signaling process can be really complex and often they can even end at the nucleus which affects gene transcription. Here, now that we have our proteins bound we're gonna have our signal transduction so the signal's gonna passed on into to the cytosol and ultimately this often ends in regulating gene transcription. Which ultimately affects the production of proteins. What do RTKs actually do in our body? Enzyme-linked receptors in general have a variety of functions but receptor tyrosine kinase is again one of the most famous and most well-known enzyme-linked receptors, and these are primarily known for their role in growth factors. Such as in regulating surface proteins called ephrins which can help guide developmental processes involved in tissue architecture, placement of nerve endings and blood vessel maturation. Other growth factors including things like nerve growth factors and platelet-derived growth factors also use RTKs. Another thing that RTKs are famous for is they can also bind hormones most famously insulin. Now what happens when RTKs fail to function properly? Since RTKs primarily regulate cell growth they can cause issues in the growth and differentiation of cells if they're not working. In fact, because of this many cancers involve mutations in RTKs. For this reason, RTKs are actually a target of many drugs that are used in chemotherapy. For example, the breast cancer drugs Herceptin is an antibody that binds and inhibits a particular RTK that is over expressed in many different breast cancers. In summary, enzyme-linked receptors essentially turn an extracellular chemical signal into enzyme activity inside the cell. Specifically the most well-known of those are receptor tyrosine kinases. These are the largest and most well-known group. The binding of a signaling molecule with an RTK activates tyrosine kinase in the cytoplasmic section of the receptor. This activity then can lodge a series of many different enzymatic reactions, it can bind different proteins which ultimately undergo complicated signal transduction generally carrying the signal to the nucleus which can then alter gene expression.
Function
Serine/threonine kinases play a role in the regulation of cell proliferation, programmed cell death (apoptosis), cell differentiation, and embryonic development.
Selectivity
While serine/threonine kinases all phosphorylate serine or threonine residues in their substrates, they select specific residues to phosphorylate on the basis of residues that flank the phosphoacceptor site, which together comprise the consensus sequence. Since the consensus sequence residues of a target substrate only make contact with several key amino acids within the catalytic cleft of the kinase (usually through hydrophobic forces and ionic bonds), a kinase is usually not specific to a single substrate, but instead can phosphorylate a whole "substrate family" which share common recognition sequences. While the catalytic domain of these kinases is highly conserved, the sequence variation that is observed in the kinome (the subset of genes in the genome that encode kinases) provides for recognition of distinct substrates. Many kinases are inhibited by a pseudosubstrate that binds to the kinase like a real substrate but lacks the amino acid to be phosphorylated. When the pseudosubstrate is removed, the kinase can perform its normal function.
EC numbers
Many serine/threonine protein kinases do not have their own individual EC numbers and use 2.7.11.1, "non-specific serine/threonine protein kinase". This entry is for any enzyme that phosphorylates proteins while converting ATP to ADP (i.e., ATP:protein phosphotransferases.)[10] 2.7.11.37 "protein kinase" was the former generic placeholder and was split into several entries (including 2.7.11.1) in 2005.[11] 2.7.11.70 "protamine kinase" was merged into 2.7.11.1 in 2004.[12]
2.7.11.- is the generic level where all serine/threonine kinases should sit in.[13]
Types
Types include those acting directly as membrane-bound receptors (Receptor protein serine/threonine kinase) and intracellular kinases participating in Signal transduction. Of the latter, types include:
| EC number | Name | Description |
|---|---|---|
| EC 2.7.11.1 | CK2, also known by the misnomer casein kinase 2 | was discovered in 1954 by Burnett and Kennedy. |
| EC 2.7.11.1 | Mos/Raf kinases | form part of the MAPKK Kinase family and are activated by growth factors. The enzyme functions to stimulate growth of cells. Raf inhibition has become the target for new anti-metastatic cancer drugs as they inhibit the MAPK cascade and reduce cell proliferation. |
| EC 2.7.11.1 | Protein Kinase B, also known as AKT kinase | The v-akt gene was identified as the oncogene of retrovirus AKT8. The gene codes for a protein kinase. Human homologs of the AKT8 oncogenic protein were identified in 1987.By 1995 it had been found that Akt kinases function as mitogen-activated kinases downstream from cell surface receptors that activate phosphoinositide 3-kinase. Three human akt genes exist. All three Akt kinases regulate cell proliferation and Akt2 is particularly important for insulin actions in cells. A major target of Akt kinases is glycogen synthase kinase-3. |
| EC 2.7.11.1 | Pelle | is a serine/threonine kinase that can phosphorylate itself, and also Tube and Toll. |
| EC 2.7.11.11 | Protein kinase A | consists of two domains, a small domain with several β sheet structures and a larger domain containing several α helices. The binding sites for substrate and ATP are located in the catalytic cleft between the domains (or lobes). When ATP and substrate bind, the two lobes rotate so that the terminal phosphate group of the ATP and the target amino acid of the substrate move into the correct positions for the catalytic reaction to take place. |
| EC 2.7.11.13 | Protein kinase C ('PKC') | is actually a family of protein kinases consisting of ~10 isozymes. They are divided into three subfamilies: conventional (or classical), novel, and atypical based on their second messenger requirements. |
| EC 2.7.11.24 | Mitogen-activated protein kinases (MAPKs) | respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, and cell survival/apoptosis. |
| EC 2.7.11.17 | Ca2+/calmodulin-dependent protein kinases or CaM kinases (CAMK) | are primarily regulated by the Ca2+/calmodulin complex. |
| EC 2.7.11.19 | Phosphorylase kinase | was in fact, the first Ser/Thr protein kinase to be discovered (in 1959 by Krebs et al.). |
Clinical significance
Serine/threonine kinase (STK) expression is altered in many types of cancer.[14] Limited benefit of serine/threonine kinase inhibitors has been demonstrated in ovarian cancer[15] but studies are ongoing to evaluate their safety and efficacy.
Serine/threonine protein kinase p90-kDa ribosomal S6 kinase (RSK) is in involved in development of some prostate cancers.[16]
Raf inhibition has become the target for new anti-metastatic cancer drugs as they inhibit the MAPK cascade and reduce cell proliferation.
See also
- Protein serine/threonine phosphatase, enzyme for reverse process.
- Pseudokinase, a protein without enzyme activity (pseudoenzyme). It can be related to proteins of this class.
- ATM serine/threonine kinase, responsible for the disorder ataxia–telangiectasia.
References
- ^ Nowakowski, J.; Cronin, C. N.; McRee, D. E.; Knuth, M. W.; Nelson, C. G.; Pavletich, N. P.; Rogers, J.; Sang, B. C.; Scheibe, D. N.; Swanson, R. V.; Thompson, D. A. (2002). "Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography". Structure. 10 (12): 1659–1667. doi:10.1016/S0969-2126(02)00907-3. PMID 12467573.
- ^ Modi, V; Dunbrack, RL (24 December 2019). "A Structurally-Validated Multiple Sequence Alignment of 497 Human Protein Kinase Domains". Scientific Reports. 9 (1): 19790. Bibcode:2019NatSR...919790M. doi:10.1038/s41598-019-56499-4. PMC 6930252. PMID 31875044.
- ^ Damuni Z, Reed LJ (1988). "Purification and properties of a protamine kinase and a type II casein kinase from bovine kidney mitochondria". Arch. Biochem. Biophys. 262 (2): 574–84. doi:10.1016/0003-9861(88)90408-0. PMID 2835010.
- ^ Baggio B, Pinna LA, Moret V, Siliprandi N (1970). "A simple procedure for the purification of rat liver phosvitin kinase". Biochim. Biophys. Acta. 212 (3): 515–7. doi:10.1016/0005-2744(70)90261-5. PMID 5456997.
- ^ Jergil B, Dixon GH (1970). "Protamine kinase from rainbow trout testis. Partial purification and characterization". J. Biol. Chem. 245 (2): 425–34. doi:10.1016/S0021-9258(18)63408-8. PMID 4312674.
- ^ Langan TA (1969). "Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo". J. Biol. Chem. 244 (20): 5763–5. doi:10.1016/S0021-9258(18)63626-9. PMID 4310608.
- ^ Takeuchi M, Yanagida M (1993). "A mitotic role for a novel fission yeast protein kinase dsk1 with cell cycle stage dependent phosphorylation and localization". Mol. Biol. Cell. 4 (3): 247–60. doi:10.1091/mbc.4.3.247. PMC 300923. PMID 8485317.
- ^ NF; Lützelberger, M; Weigmann, H; Klingenhoff, A; Shenoy, S; Käufer, NF (1997). "Functional analysis of the fission yeast Prp4 protein kinase involved in pre-mRNA splicing and isolation of a putative mammalian homologue". Nucleic Acids Res. 25 (5): 1028–35. doi:10.1093/nar/25.5.1028. PMC 146536. PMID 9102632.
- ^ Wang Y, Hofmann TG, Runkel L, Haaf T, Schaller H, Debatin K, Hug H (2001). "Isolation and characterization of cDNAs for the protein kinase HIPK2". Biochim. Biophys. Acta. 1518 (1–2): 168–72. doi:10.1016/S0167-4781(00)00308-0. PMID 11267674.
- ^ "ENZYME - 2.7.11.1 non-specific serine/threonine protein kinase". enzyme.expasy.org. Retrieved 2023-12-25.
- ^ "KEGG ENZYME: 2.7.1.37". www.genome.jp. Retrieved 2023-12-25.
- ^ "KEGG ENZYME: 2.7.1.70". www.genome.jp. Retrieved 2023-12-25.
- ^ "EC 2.7.11". iubmb.qmul.ac.uk. Retrieved 2023-12-25.
- ^ Capra, Maria; Nuciforo, Paolo Giovanni; Confalonieri, Stefano; Quarto, Micaela; Bianchi, Marco; Nebuloni, Manuela; Boldorini, Renzo; Pallotti, Francesco; Viale, Giuseppe; Gishizky, Mikhail L.; Draetta, Giulio F.; Fiore, Pier Paolo Di (15 August 2006). "Frequent Alterations in the Expression of Serine/Threonine Kinases in Human Cancers". Cancer Research. 66 (16): 8147–8154. doi:10.1158/0008-5472.CAN-05-3489. PMID 16912193.
- ^ Ciccone, Marcia A.; Maoz, Asaf; Casabar, Jennifer K.; Machida, Hiroko; Mabuchi, Seiji; Matsuo, Koji (2 July 2016). "Clinical outcome of treatment with serine-threonine kinase inhibitors in recurrent epithelial ovarian cancer: a systematic review of literature". Expert Opinion on Investigational Drugs. 25 (7): 781–796. doi:10.1080/13543784.2016.1181748. PMC 7534810. PMID 27101098.
- ^ Clark, D. E.; Errington, T. M.; Smith, J. A.; Frierson, H. F.; Weber, M. J.; Lannigan, D. A. (15 April 2005). "The Serine/Threonine Protein Kinase, p90 Ribosomal S6 Kinase, Is an Important Regulator of Prostate Cancer Cell Proliferation". Cancer Research. 65 (8): 3108–3116. doi:10.1158/0008-5472.CAN-04-3151. PMID 15833840.
External links
- protein-serine-threonine+kinases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- KinCore (Kinase Conformational Resource)

