| |
| |
| Names | |
|---|---|
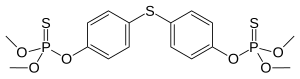
| Preferred IUPAC name
O-[4-({4-[(Dimethoxyphosphorothioyl)oxy]phenyl}sulfanyl)phenyl] O,O-dimethyl phosphorothioate | |
| Other names
[4-(4-dimethoxyphosphinothioyloxyphenyl)sulfanylphenoxy]-
dimethoxy-sulfanylidene-phosphorane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.175 |
| KEGG | |
| MeSH | Temefos |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H20O6P2S3 | |
| Molar mass | 466.46 g·mol−1 |
| Appearance | white, crystalline solid[1] |
| Density | 1.32 g cm−3 |
| Melting point | 30 °C (86 °F; 303 K) |
| Boiling point | 120–125[1] °C (248–257 °F; 393–398 K) (decomposes) |
| insoluble[1] | |
| Vapor pressure | 0.00000007 mmHg (20°C)[1] |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
15 mg/m3[1] |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Temefos or temephos (trade name Abate) is an organophosphate larvicide used to treat water infested with disease-carrying insects[2] including mosquitoes, midges, and black fly larvae.
As with other organophosphates, temephos affects the central nervous system through inhibition of cholinesterase. In larvae, this results in death before reaching the adult stage.
In the developing world where the vector-borne disease dengue fever is endemic, temephos is widely used and applied by both private and public pest control in areas of standing water where the Aedes aegypti mosquito breeds in order to reduce the population of this disease-carrying insect.[3] Temephos is also used in the Guinea worm eradication program to kill water fleas that carry guinea worm larvae.
Resistance to temephos by A. aegypti has been seen in Brazil. The Brazilian Aedes aegypti resistance monitoring program detected temephos resistance in A. aegypti populations from several localities in the country in 1999 (Funasa 2000, Lima et al. 2003). In 1999, mosquitoes from the city of Rio de Janeiro were already resistant to temephos.[4]
YouTube Encyclopedic
-
1/3Views:1 360356659
-
Bioinseticida - Momento Ambiental
-
Community Medicine 909 e Insecticide Malathion
-
Community Medicine 129 e Different Types of Mosquito Difference between Anopheles Aedes Culex
Transcription
References
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0589". National Institute for Occupational Safety and Health (NIOSH).
- ^ Abate Product Information
- ^ New York Times article covering its application in Africa
- ^ Lima JB, Da-Cunha MP, Da Silva RC, et al. (2003). "Resistance of Aedes aegypti to organophosphates in several municipalities in the State of Rio de Janeiro and Espírito Santo, Brazil". Am. J. Trop. Med. Hyg. 68 (3): 329–33. doi:10.4269/ajtmh.2003.68.329. PMID 12685640.
External links
- NIOSH Pocket Guide to Chemical Hazards
- International Chemical Safety Cards
- Official product site
- Temefos in the Pesticide Properties DataBase (PPDB)
Bibliography
- Dejous C & Elouard J.M (1977) Action de l'abate sur les invertébérs aquatiques ; cinétique de décrochement à court et moyen terme (in French).
