| |
| |
| Names | |
|---|---|
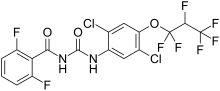
| IUPAC name
1-[2,5-Dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-3-(2,6-difluorobenzoyl)urea
| |
| Other names
N-[[[2,5-Dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl]amino]carbonyl]-2,6-difluorobenzamide
Fluphenacur U.S. EPA PC Code: 118205 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.101.025 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H8Cl2F8N2O3 | |
| Molar mass | 511.15 g·mol−1 |
| Melting point | 174 °C (345 °F; 447 K) |
| Pharmacology | |
| QP53BC01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lufenuron is the active ingredient in the veterinary flea control medication Program, and one of the two active ingredients in the flea, heartworm, ringworm and anthelmintic medicine milbemycin oxime/lufenuron (Sentinel).
Lufenuron is stored in the animal's body fat and transferred to adult fleas through the host's blood when they feed. Adult fleas transfer it to their growing eggs through their blood, and to hatched larvae feeding on their excrement. It does not kill adult fleas.[citation needed]
Lufenuron, a benzoylurea pesticide, inhibits the production of chitin in insects. Without chitin, a larval flea will never develop a hard outer shell (exoskeleton). With its inner organs exposed to air, the insect dies from dehydration soon after hatching or molting (shedding its old, smaller shell).[citation needed]
Lufenuron is also used to fight fungal infections, since fungus cell walls are about one third chitin.[1]
Lufenuron is also sold as an agricultural pesticide for use against lepidopterans, eriophyid mites, and western flower thrips. It is an effective antifungal in plants.[2]
YouTube Encyclopedic
-
1/3Views:3 2501 22229 011
-
Lufenuron Treats Systemic Fungal Infection: Proof Exposed!
-
How Can I Break Candida Chitin?
-
5 Stages of Candida Overgrowth
Transcription
References
- ^ Ben-Ziony, Yair; Arzi, Boaz (2000). "Use of lufenuron for treating fungal infections of dogs and cats: 297 cases (1997-1999)". Journal of the American Veterinary Medical Association. 217 (10): 1510–3. doi:10.2460/javma.2000.217.1510. PMID 11128542.
- ^ Paranjape, Kalyani; Gowariker, Vasant; Krishnamurthy, V N (2014). The Pesticide Encyclopedia. Wallingford, Oxfordshire UK Boston, MA: CABI. p. 283. ISBN 978-1-78064-014-3. Retrieved Nov 22, 2018.
Lufenuron, a chitin synthesis inhibitor, is an effective agent against lepidopteran members in crops, eriophid mites and western flower thrips. In non-crop situations too, lufenuron is effective against fleas on animals and on cockroaches in households. This chemical also acts as an anti-fungal agent in plants. ... According to the WHO, lufenuron is a Class III toxin (slightly hazardous). It is safe on mammals, since it is not broken down by the liver or kidneys.
External links
- Lufenuron in the Pesticide Properties DataBase (PPDB)
