| |

| |
| Names | |
|---|---|
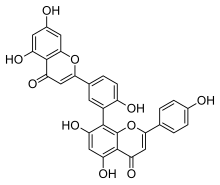
| IUPAC name
(4′,5,7-Trihydroxyflavone)-(3′→8)-(4′,5,7-trihydroxyflavone)
| |
| Systematic IUPAC name
8-[5-(5,7-Dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Didemethyl-ginkgetin
3′,8″-Biapigenin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H18O10 | |
| Molar mass | 538.464 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amentoflavone is a biflavonoid (bis-apigenin coupled at 8 and 3′ positions, or 3′,8″-biapigenin) constituent of a number of plants including Ginkgo biloba, Chamaecyparis obtusa (hinoki), Biophytum sensitivum, Selaginella tamariscina,[1] Hypericum perforatum (St. John's Wort)[2] and Xerophyta plicata.[3]
Amentoflavone can interact with many medications by being a potent inhibitor of CYP3A4 and CYP2C9, which are enzymes responsible for the metabolism of some drugs in the body.[4] It is also an inhibitor of human cathepsin B.[2]
Amentoflavone has a variety of in vitro activities including antimalarial activity,[5] anticancer activity (which may, at least in part, be mediated by its inhibition of fatty acid synthase),[6][7][8] and antagonist activity at the κ-opioid receptor (Ke = 490 nmol L−1)[9] as well as activity at the allosteric benzodiazepine site of the GABAA receptor as a negative allosteric modulator.[10]
YouTube Encyclopedic
-
1/4Views:218 9323 949348996
-
What is Ginkgo Biloba? – The Benefits of Ginkgo Biloba – Dr.Berg
-
Krambilan (Biophytum sensitivum) - part 1
-
24.古湘儒-銀在最終點-銀杏萃取物之穗花杉雙黃酮 Amentoflavone 的抗癌功效
-
Lecture 12, concept 02: Understand your drug target - SARS-CoV-2 as an example
Transcription
See also
References
- ^ Xiong X (22 December 2021). "Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid". Frontiers in Pharmacology. 12. doi:10.3389/fphar.2021.768708. PMC 8727548. PMID 35002708.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b Pan X, Tan N, Zeng G, Zhang Y, Jia R (2005). "Amentoflavone and its derivatives as novel natural inhibitors of human Cathepsin B". Bioorg. Med. Chem. 13 (20): 5819–5825. doi:10.1016/j.bmc.2005.05.071. PMID 16084098.
- ^ Williams CA, Harborne JB, Tomas-Barberan A F (1987). "Biflavonoids in the primitive monocots Isophysis tasmanica and Xerophyta plicata". Phytochemistry. 26 (9): 2553. Bibcode:1987PChem..26.2553W. doi:10.1016/S0031-9422(00)83875-3.
- ^ Kimura, Y, Ito, H, Ohnishi, R, Hatano, T (2010). "Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity". Food Chem. Toxicol. 48 (1): 429–435. doi:10.1016/j.fct.2009.10.041. PMID 19883715.
- ^ "Inhibitors of Plasmodium falciparum M1- Family Alanyl Aminopeptidase (M1AAP)".
- ^ Lee, JS, Lee, MS, Oh, WK, Sul, JY (2009). "Fatty acid synthase inhibition by amentoflavone induces apoptosis and antiproliferation in human breast cancer cells" (PDF). Biol. Pharm. Bull. 32 (8): 1427–1432. doi:10.1248/bpb.32.1427. PMID 19652385.
- ^ Wilsky, S, Sobotta, K, Wiesener, N, Pilas, J, Althof, N, Munder, T, Wutzler, P, Henke, A (2012). "Inhibition of fatty acid synthase by amentoflavone reduces coxsackievirus B3 replication". Arch. Virol. 157 (2): 259–269. doi:10.1007/s00705-011-1164-z. PMID 22075919. S2CID 254054659.
- ^ Lee, JS, Sul, JY, Park, JB, Lee, MS, Cha, EY, Song, IS, Kim, JR, Chang, ES (2013). "Fatty Acid Synthase Inhibition by Amentoflavone Suppresses HER2/neu(erbB2) Oncogene in SKBR3 Human Breast Cancer Cells". Phytother. Res. 27 (5): 713–720. doi:10.1002/ptr.4778. PMID 22767439. S2CID 26035868.
- ^ Katavic PL, Lamb K, Navarro H, Prisinzano TE (2007). "Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships". J. Nat. Prod. 70 (8): 1278–1282. doi:10.1021/np070194x. PMC 2265593. PMID 17685652.
- ^ Hanrahan JR, Chebib M, Davucheron NL, Hall BJ, Johnston GA (2003). "Semisynthetic preparation of amentoflavone: A negative modulator at GABA(A) receptors". Bioorg. Med. Chem. Lett. 13 (14): 2281–2284. doi:10.1016/s0960-894x(03)00434-7. PMID 12824018.
External links
 Media related to Amentoflavone at Wikimedia Commons
Media related to Amentoflavone at Wikimedia Commons
