 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
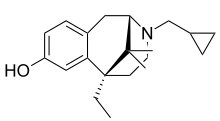
| Formula | C20H29NO |
| Molar mass | 299.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gemazocine (R-15,497), also known as cyclogemine, is a non-selective opioid antagonist of the benzomorphan class.[1][2][3] It may have partial agonist properties at some of the opioid receptors, such as at the kappa receptor (as it induces dysphoric effects in humans), but seems to be generally antagonistic in its actions.[4]
References
- ^ Macdonald F (1997). Dictionary of Pharmacological Agents. CRC Press. p. 955. ISBN 978-0-412-46630-4. Retrieved 22 April 2012.
- ^ Gelders YG, de Ranter CJ, Schenk H (1979). "Structural Studies of Substituted 6,7-Benzomorphan Compounds. I. The Absolute Configuration of (−)-2-Cyclopropylmethyl-2'-hydroxy-5-ethyl-9,9-dimethyl-6,7-benzomorphan (Gemazocine) Hydrobromide". Acta Crystallographica B. 35 (3): 699–703. Bibcode:1979AcCrB..35..699G. doi:10.1107/S0567740879004477.
- ^ Verlinde C, De Ranter C (August 1988). "Assessment of the kappa-opioid activity of a series of 6,7-benzomorphans in the rabbit vas deferens". European Journal of Pharmacology. 153 (1): 83–87. doi:10.1016/0014-2999(88)90590-0. PMID 2850928.
- ^ Freye E, Hartung E, Schenk GK (May 1983). "Bremazocine: an opiate that induces sedation and analgesia without respiratory depression". Anesthesia and Analgesia. 62 (5): 483–488. doi:10.1213/00000539-198305000-00005. PMID 6301311.
