| |
| Names | |
|---|---|
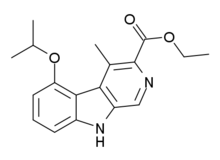
| Preferred IUPAC name
Ethyl 4-methyl-5-[(propan-2-yl)oxy]-9H-pyrido[3,4-b]indole-3-carboxylate | |
| Other names
ZK-93426
Ethyl 5-isopropoxy-4-methyl-9H-β-carboline-3-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H20N2O3 | |
| Molar mass | 314.336 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
ZK-93426 (ethyl-5-isopropoxy-4-methyl-beta-carboline-3-carboxylate) is a drug from the beta-carboline family. It acts as a weak partial inverse agonist of benzodiazepine receptors,[1] meaning that it causes the opposite effects to the benzodiazepine class of drugs and has anxiogenic properties,[2] although unlike most benzodiazepine antagonists it is not a convulsant and actually has weak anticonvulsant effects.[3] In human tests it produced alertness, restlessness and feelings of apprehension[clarification needed], and reversed the effect of the benzodiazepine lormetazepam.[4][5] It was also shown to produce nootropic effects[6][7] and increased release of acetylcholine.[8][9]
YouTube Encyclopedic
-
1/1Views:93 822
-
This is it This is it This is it!!!!
Transcription
See also
References
- ^ Camerman, A; Mastropaolo, D; Hempel, A; Camerman, N (2005). "Ethyl 5-isopropoxy-4-methyl-beta-carboline-3-carboxylate: structural determinants of benzodiazepine-receptor antagonism". Acta Crystallographica C. 61 (Pt 4): o265–6. Bibcode:2005AcCrC..61O.265C. doi:10.1107/S0108270105005457. PMID 15805647.
- ^ File, SE; Pellow, S; Jensen, LH (1986). "Actions of the beta-carboline ZK 93426 in an animal test of anxiety and the holeboard: interactions with Ro 15-1788". Journal of Neural Transmission. 65 (2): 103–14. doi:10.1007/BF01256486. PMID 3009709. S2CID 7230560.
- ^ Jensen, LH; Petersen, EN; Braestrup, C; Honoré, T; Kehr, W; Stephens, DN; Schneider, H; Seidelmann, D; Schmiechen, R (1984). "Evaluation of the beta-carboline ZK 93 426 as a benzodiazepine receptor antagonist". Psychopharmacology. 83 (3): 249–56. doi:10.1007/BF00464789. PMID 6089247. S2CID 37990146.
- ^ Dorow, R; Duka, T; Höller, L; Sauerbrey, N (1987). "Clinical perspectives of beta-carbolines from first studies in humans". Brain Research Bulletin. 19 (3): 319–26. doi:10.1016/0361-9230(87)90100-6. PMID 2890423. S2CID 54287050.
- ^ Duka, T; Goerke, D; Dorow, R; Höller, L; Fichte, K (1988). "Human studies on the benzodiazepine receptor antagonist beta-carboline ZK 93 426: antagonism of lormetazepam's psychotropic effects". Psychopharmacology. 95 (4): 463–71. doi:10.1007/bf00172956. PMID 2905500. S2CID 25383323.
- ^ Duka, T; Edelmann, V; Schütt, B; Dorow, R (1988). "β-Carbolines as Tools in Memory Research: Human Data with the β-Carboline ZK 93426". Benzodiazepine Receptor Ligands, Memory and Information Processing. Vol. 6. pp. 246–60. doi:10.1007/978-3-642-73288-1_18. ISBN 978-3-642-73290-4. PMID 3064085.
{{cite book}}:|journal=ignored (help) - ^ Duka, T; Ott, H; Rohloff, A; Voet, B (1996). "The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills". Psychopharmacology. 123 (4): 361–73. doi:10.1007/BF02246647. PMID 8867876. S2CID 36950692.
- ^ Moore, H; Sarter, M; Bruno, JP (1992). "Age-dependent modulation of in vivo cortical acetylcholine release by benzodiazepine receptor ligands". Brain Research. 596 (1–2): 17–29. doi:10.1016/0006-8993(92)91527-L. PMID 1334777. S2CID 39356368.
- ^ Moore, H; Sarter, M; Bruno, JP (1993). "Bidirectional modulation of stimulated cortical acetylcholine release by benzodiazepine receptor ligands". Brain Research. 627 (2): 267–74. doi:10.1016/0006-8993(93)90330-P. PMID 8298971. S2CID 29549067.
