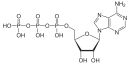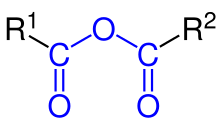
An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom.[1] A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride.[2] Thus, (CH3CO)2O is called acetic anhydride. Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").[3]
One or both acyl groups of an acid anhydride may also be derived from another type of organic acid, such as sulfonic acid or a phosphonic acid. One of the acyl groups of an acid anhydride can be derived from an inorganic acid such as phosphoric acid. The mixed anhydride 1,3-bisphosphoglyceric acid, an intermediate in the formation of ATP via glycolysis,[4] is the mixed anhydride of 3-phosphoglyceric acid and phosphoric acid. Acidic oxides are also classified as acid anhydrides.
YouTube Encyclopedic
-
1/3Views:31 7301 767147 518
-
Preparation of acid anhydrides | Carboxylic acids and derivatives | Organic chemistry | Khan Academy
-
Nomenclature of Acid Anhydrides
-
Amides, anhydrides, esters, and acyl chlorides | Organic chemistry | Khan Academy
Transcription
Voiceover: Here's another carboxylic acid derivative so, this is an acid anhydride over here on the right. And we can form those from carboxylic acids. So if we start with the carboxylic acid, and our first step, add a base, like sodium hydroxide, and our second step, add an acyl chloride, then we'd form our acid anhydride as our product. Now if you think about a mechanism, sodium hydroxide's a base. The hydroxide anion is gonna take this proton, leaving these electrons behind on the oxygen. There are already two lone pairs of electrons on the oxygen to start with, so if we go ahead and draw the product, we would form a carboxylate anion. So, this oxygen right here would have three lone pairs of electrons on it, like that. And so if we follow some of those electrons, this would have a -1 formal charge, and if we put these electrons in magenta, those electrons come off onto our oxygen to form our carboxylate anion. And that's gonna function as our nucleophile. So in the second step, we add our acyl chloride. I'm just gonna go ahead and draw in our acyl chloride. It's going to be our electrophile, and let's think about why here. We have the oxygen withdrawing electron density from this carbon, because oxygen is more electronegative than carbon, so we have that. And then we also have this chlorine doing it as well. Chlorine is more electronegative than carbon as well, so we have these two things withdrawing electron density, and so this carbon is definitely electrophilic, right here. And so we have a nucleophile that's going to attack our electrophile, so our nucleophile attacks our electrophile, these electrons kick off onto our oxygen, and we can go ahead and show the result of our nucleophilic attack. So we would now have this carbon double-bonded to this oxygen, and then this oxygen is now bonded to this carbon, and then we have an oxygen up here, with three lone pairs of electrons, -1 formal charge. We still have this carbon bonded to a chlorine, and we still have an R prime group here, like that. So following some electrons, let's go ahead and put in these lone pairs here, the electrons in magenta form the bond between the oxygen and the carbon, and then we could say that these electrons in here moved off onto our oxygen, to give that oxygen a -1 formal charge. When we think about the next step, we know that the chloride anion is an excellent leaving group. So, if these electrons in blue move in here to reform our double bond, then these electrons would kick off onto the chlorine to form the chloride anion, which we know is a stable on its own, as a leaving group. And so, that forms our acid anhydride, so let's go ahead and draw in the final structure here. So we would have our oxygen, with lone pairs of electrons on our oxygen. We would have this bond. We reformed our carbonyl like that, and then the chloride anion was our leaving group, so now we have an R prime group, and so we've formed our acid anhydride. And so we could make these R groups the same, or we could make them different, and so this is a good way of forming a mixed anhydride, as well as one that is symmetrical. So let's look at an example. So let's take acetic acid and we're gonna do two different reactions with acetic acid. So the first thing we're gonna do, is add thionyl chloride to acetic acid, and we've seen that the addition of thionyl chloride converts a carboxylic acid into an acyl halide. So let's go ahead and show the conversion of that carboxylic acid into the corresponding acyl halide. And then we could take that acetic acid, and in a separate reaction, we could add sodium hydroxide, and the hydroxide takes this proton, which leaves these electrons behind on the oxygen. So let's scoot up a little bit, so we can see the mechanism that we talked about before. And so this would form sodium acetate, draw in our lone pairs of electrons on this oxygen, -1 formal charge, and a plus like that. So this oxygen already had two lone pairs of electrons. And so now you have the situation that we talked about up here. Your carboxylate anion functions as a nucleophile, attacks your electrophilic carbon on your acyl chloride. So we could show these electrons attacking this carbon, these electrons kick off onto the oxygen, and then when those electrons move back in, to reform your double bond, these electrons would kick off onto your chlorine, and then so we can go ahead and draw our product. So just thinking about what happens in this mechanism, we can go ahead and draw our products, which would be a symmetical anhydride, so we would have our oxygen right here, and then we would have our group over here. So thinking about the R groups this time, so this R group is a methyl group, and then we could think about this oxygen being this oxygen, and then this portion of the acyl chloride is this portion for our final product for our acid anhydride. And so this is acetic anhydride, which is the one that's used most commonly in an undergraduate lab. And so this is a nice way of preparing acid anhydrides. Let's look at another way to form acetic anhydride. You could start with two carboxylic acids, and this would be acetic acid and acetic acid, so the same carboxylic acid, and apply high heat, and this time you think about a dehydration reaction, so you could think about losing an OH from one, and a hydrogen from the other, to form water, so you could think about losing a water here, and your dehydration reaction. And then you can stick those portions of the molecule together, so you could take this portion, and then this portion, and put them together, and you can see that that is once again acetic anhydride, so let's go ahead and draw that. So we would form acetic anhydride here by dehydration. So this way of doing it is not always the best way, it works for acetic acid, but it doesn't work for most carboxylic acids. Here's one more case where it can work, if you have a situation like this. This is phthalic acid, so it's a dicarboxylic acid, And if you apply heat to it, you don't need as high of a heat as you need for the previous reaction, this heat is higher than this one, but you can once again form an anhydride. So if you think about a dehydration, losing OH from one, and H from the other, and then we can go ahead and draw the product here, so we would form our benzene ring, and then we would form our anhydride like this. So once again, loss of water. So the name of this acid anhydride would be phthalic anhydride because it's derived from phthalic acid. So this is a good way to form five- or six-membered rings. In this case, we have a five-membered ring. We have a carbon, an oxygen, a carbon, a carbon, and a carbon, so we have a five-membered ring this time. It also works for six-membered rings.
Nomenclature
The nomenclature of organic acid anhydrides is derived from the names of the constituent carboxylic acids. In symmetrical acid anhydrides, only the prefix of the original carboxylic acid is used and the suffix "anhydride" is added. For most unsymmetrical acid anhydrides - also called mixed anhydrides- the prefixes from both acids reacted are listed before the suffix, e.g., benzoic propanoic anhydride.[5]
Preparation
Organic acid anhydrides are prepared in industry by diverse means. Acetic anhydride is mainly produced by the carbonylation of methyl acetate.[6] Maleic anhydride is produced by the oxidation of benzene or butane. Laboratory routes emphasize the dehydration of the corresponding acids. The conditions vary from acid to acid, but phosphorus pentoxide is a common dehydrating agent:
- 2 CH3COOH + P4O10 → CH3C(O)OC(O)CH3 + "P4O9(OH)2"
Acid chlorides are also effective precursors:[7]
- CH3C(O)Cl + HCO2Na → HCO2COCH3 + NaCl
Mixed anhydrides containing the acetyl group are prepared from ketene:
- RCO2H + H2C=C=O → RCO2C(O)CH3
Reactions
Acid anhydrides are a source of reactive acyl groups, and their reactions and uses resemble those of acyl halides. In reactions with protic substrates, the reactions afford equal amounts of the acylated product and the carboxylic acid:
- RC(O)OC(O)R + HY → RC(O)Y + RCO2H
for HY = HOR (alcohols), HNR'2 (ammonia, primary, secondary amines), aromatic ring (see Friedel-Crafts acylation).
Acid anhydrides tend to be less electrophilic than acyl chlorides, and only one acyl group is transferred per molecule of acid anhydride, which leads to a lower atom efficiency. The low cost, however, of acetic anhydride makes it a common choice for acetylation reactions.
Applications and occurrence of acid anhydrides
- Illustrative acid anhydrides
-
Acetic anhydride is produced on a large scale for many applications.
-
Naphthalenetetracarboxylic dianhydride, a building block for complex organic compounds, is an example of a dianhydride.
-
Maleic anhydride is a cyclic anhydride, widely used to make industrial coatings.
-
ATP in its protonated form is an anhydride derived from phosphoric acid.
-
The "mixed anhydride" 1,3-bisphosphoglyceric acid occurs widely in metabolic pathways.
-
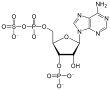
3'-Phosphoadenosine-5'-phosphosulfate (PAPS) is a mixed anhydride of sulfuric and phosphoric acids and is the most common coenzyme in biological sulfate transfer reactions.
Acetic anhydride is a major industrial chemical widely used for preparing acetate esters, e.g. cellulose acetate. Maleic anhydride is the precursor to various resins by copolymerization with styrene. Maleic anhydride is a dienophile in the Diels-Alder reaction.[8]
Dianhydrides, molecules containing two acid anhydride functions, are used to synthesize polyimides and sometimes polyesters[9] and polyamides.[10] Examples of dianhydrides: pyromellitic dianhydride (PMDA), 3,3’, 4,4’ - oxydiphtalic dianhydride (ODPA), 3,3’, 4,4’-benzophenone tetracarboxylic dianhydride (BTDA), 4,4’-diphtalic (hexafluoroisopropylidene) anhydride (6FDA), benzoquinonetetracarboxylic dianhydride, ethylenetetracarboxylic dianhydride. Polyanhydrides are a class of polymers characterized by anhydride bonds that connect repeat units of the polymer backbone chain.
Biological occurrence
Natural products containing acid anhydrides have been isolated from animals, bacteria and fungi.[11][12][13] Examples include cantharidin from species of blister beetle, including the Spanish fly, Lytta vesicatoria, and tautomycin, from the bacterium Streptomyces spiroverticillatus. The maleidride family of fungal secondary metabolites, which possess a wide range of antibiotic and antifungal activity, are alicyclic compounds with maleic anhydride functional groups.[14] A number of proteins in prokaryotes[15] and eukaryotes[16] undergo spontaneous cleavage between the amino acid residues aspartic acid and proline via an acid anhydride intermediate. In some cases, the anhydride may then react with nucleophiles of other cellular components, such as at the surface of the bacterium Neisseria meningitidis or on proteins localized nearby.[17]
Analogues
Nitrogen

Imides are structurally related analogues, where the bridging oxygen is replaced by nitrogen. They are similarly formed by the condensation of dicarboxylic acids with ammonia. The replacement of all oxygen atoms with nitrogen gives imidines, these are a rare functional group which are very prone to hydrolysis.
Sulfur
Sulfur can replace oxygen, either in the carbonyl group or in the bridge. In the former case, the name of the acyl group is enclosed in parentheses to avoid ambiguity in the name,[2] e.g., (thioacetic) anhydride (CH3C(S)OC(S)CH3). When two acyl groups are attached to the same sulfur atom, the resulting compound is called a thioanhydride,[2] e.g., acetic thioanhydride ((CH3C(O))2S).
See also
- Base anhydride
- Benzoyl peroxide - structurally similar but chemically very different
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "acid anhydrides". doi:10.1351/goldbook.A00072
- ^ a b c Panico, R.; Powell, W. H.; Richer, J. C., eds. (1993). "Recommendation R-5.7.7". A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. pp. 123–25. ISBN 0-632-03488-2.
- ^ "Nomenclature of Anhydrides". 8 November 2013.
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ "Nomenclature of Anhydrides". 8 November 2013.
- ^ Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. "Eastman Chemical Company Acetic Anhydride Process" Catalysis Today (1992), volume 13, pp.73-91. doi:10.1016/0920-5861(92)80188-S
- ^ Lewis I. Krimen (1988). "Acetic Formic Anhydride". Organic Syntheses; Collected Volumes, vol. 6, p. 8.
- ^ Heimo Held, Alfred Rengstl, Dieter Mayer "Acetic Anhydride and Mixed Fatty Acid Anhydrides" Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_065
- ^ Chiang, Wen-Yen; Chiang, Wen-Chang (1988-05-05). "Condensation polymerization of multifunctional monomers and properties of related polyester resins. II. Thermal properties of polyester—imide varnishes". Journal of Applied Polymer Science. 35 (6): 1433–1439. doi:10.1002/app.1988.070350603.
- ^ Faghihi, Khalil; Ashouri, Mostafa; Hajibeygi, Mohsen (2013-10-25). "High Temperature and Organosoluble Poly(amide-imide)s Based on 1,4-Bis[4-aminophenoxy]butane and Aromatic Diacids by Direct Polycondensation: Synthesis and Properties". High Temperature Materials and Processes. 32 (5): 451–458. doi:10.1515/htmp-2012-0164. ISSN 2191-0324.
- ^ Saleem, Muhammad; Hussain, Hidayat; Ahmed, Ishtiaq; Draeger, Siegfried; Schulz, Barbara; Meier, Kathrin; Steinert, Michael; Pescitelli, Gennaro; Kurtán, Tibor; Flörke, Ulrich; Krohn, Karsten (February 2011). "Viburspiran, an Antifungal Member of the Octadride Class of Maleic Anhydride Natural Products". European Journal of Organic Chemistry. 2011 (4): 808–812. doi:10.1002/ejoc.201001324.
- ^ Han, Chunguang; Furukawa, Hiroyuki; Tomura, Tomohiko; Fudou, Ryosuke; Kaida, Kenichi; Choi, Bong-Keun; Imokawa, Genji; Ojika, Makoto (24 April 2015). "Bioactive Maleic Anhydrides and Related Diacids from the Aquatic Hyphomycete Tricladium castaneicola". Journal of Natural Products. 78 (4): 639–644. doi:10.1021/np500773s. PMID 25875311.
- ^ Heard, David M.; Tayler, Emyr R.; Cox, Russell J.; Simpson, Thomas J.; Willis, Christine L. (3 January 2020). "Structural and synthetic studies on maleic anhydride and related diacid natural products" (PDF). Tetrahedron. 76 (1): 130717. doi:10.1016/j.tet.2019.130717. hdl:1983/53998d06-9017-4cfb-822b-c6453348000a. S2CID 209714625.
- ^ Chen, Xiaolong; Zheng, Yuguo; Shen, Yinchu (May 2007). "Natural Products with Maleic Anhydride Structure: Nonadrides, Tautomycin, Chaetomellic Anhydride, and Other Compounds". Chemical Reviews. 107 (5): 1777–1830. doi:10.1021/cr050029r. PMID 17439289.
- ^ Kuban, Vojtech (2020). "Structural Basis of Ca 2+-Dependent Self-Processing Activity of Repeat-in-Toxin Proteins". mBio. 11 (2): e00226-20. doi:10.1128/mBio.00226-20. PMC 7078468. PMID 32184239.
- ^ Bell, Christian (2013). "Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub". Science. 341 (6141): 77–80. Bibcode:2013Sci...341...77B. doi:10.1126/science.1232322. PMC 4730555. PMID 23744777.
- ^ Scheu, Arne (2021). "NeissLock provides an inducible protein anhydride for covalent targeting of endogenous proteins". Nature Communications. 12 (1): 717. Bibcode:2021NatCo..12..717S. doi:10.1038/s41467-021-20963-5. PMC 7846742. PMID 33514717.