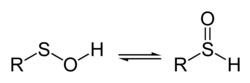
In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula R−S−OH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids (R−S(=O)OH) and sulfonic acids (R−S(=O)2OH), respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.
YouTube Encyclopedic
-
1/3Views:3 989 8408 4412 522
-
Why do we cry? The three types of tears - Alex Gendler
-
Why do we CRY ? Why Onions makes you cry while chopping ? | Educational Videos | CleverFacts
-
Cut onions with no tears
Transcription
Our story is about a girl named Iris. Iris is very sensitive. So much that she is always in tears. She cries when she's sad, when she's happy, and even tears up when things just get to her. She has special lacrimal glands to make new tears and special tubes, called lacrimal puncta, to drain old ones away. And she cries so much that she goes through ten ounces of tears per day, thirty gallons a year! In fact, if you look closely, you'll see that she's crying a little bit all the time. The basal tears that Iris constantly produces form a thin coating of three layers that cover her and keep dirt and debris away. Right next to Iris is the mucus layer, which keeps the whole thing fastened to her. On top of it is the aqueous layer, which keeps Iris hydrated, repels invasive bacteria, and protects her skin, or cornea, from damage. And, finally, there is the lipid layer, an oily outer film that keeps the surface smooth for Iris to see through, and prevents the other layers from evaporating. Normally, Iris goes about her day without really noticing the basal tears doing their thing. That's kind of their whole point. But one day, she meets a girl named Onion. Iris is immediately smitten. Onion looks gorgeous in her bright purple jacket, and she smells terrific. So, Iris invites Onion to her house for dinner, but when she comes in and takes off her jacket, something terrible happens. You see, when Onion's jacket is removed, a chemical reaction happens, converting the sulfoxides that make her smell so great into sulfenic acid, which then becomes a nasty substance with a long name: syn-Propanethial S-oxide. The gas stings Iris, and suddenenly, she can't help it, she starts weeping uncontrollably. These reflex tears are different from the basal tears that Iris is used to. Because they're designed to wash away harmful substances, or particles, they're released in much larger amounts, and their aqueous layer contains more antibodies to stop any microorganisms that may be trying to get in, as well. Both Iris and Onion are devastated. They know they can't continue their relationship if Iris is going to hurt and cry every time Onion takes off her jacket. So, they decide to break up. As Onion walks out the door, Iris stops crying. And immediately starts again. Only now, she's not crying reflex tears but emotional tears. When someone is either too sad or too happy, it feels like a loss of control, which can be dangerous. So, emotional tears are sent in to stabilize the mood as quickly as possible, along with other physical reactions, such as an increased heart rate and slower breathing. But scientists still aren't sure exactly how or why the tears themselves are helpful. They may be a social mechanism to elicit sympathy or show submission. But some studies have also found that emotional tears contain higher levels of stress hormones, such as ACTH and enkephalin, an endorphin and natural pain killer. In this case, emotional tears are also directly calming Iris down, as well as signaling her emotional state to others. Sorry things didn't work out with Onion, Iris, but don't worry. As long as you have all three kinds of tears working to keep you balanced and healthy, it will get better. You'll see.
Properties
In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH3SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy (rotational spectroscopy) to be CH3–S–O–H.[1] Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form thiosulfinates, RS(O)SR, such as allicin from garlic. Through the use of X-ray crystallography, the structure of such stabilized sulfenic acids were shown to be R–S–O–H.[2][3] The stable, sterically hindered sulfenic acid 1-triptycenesulfenic acid has been found to have a pKa of 12.5 and an O–H bond-dissociation energy (bde) of 71.9 ± 0.3 kcal/mol, which can be compared to a pKa of ≥14 and O–H BDE of ~88 kcal/mol for the (valence) isoelectronic hydroperoxides, ROOH.[4]
Formation and occurrence
Peroxiredoxins
Peroxiredoxins are ubiquitous and abundant enzymes that detoxify peroxides. They function by the conversion of a cysteine residue to a sulfenic acid. The sulfenic acid then converts to a disulfide by reaction with another residue of cysteine.[5]
Garlic and onions
Sulfenic acids are produced by the enzymatic decomposition of alliin and related compounds following tissue damage to garlic, onions, and other plants of the genus Allium. 1-Propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, the lachrymatory factor synthase, giving syn-propanethial-S-oxide.[6] 2-Propenesulfenic acid, formed from allicin, is thought to be responsible for garlic’s potent antioxidant activity.[7] Mass spectrometry with a DART ion source were used to identify 2-propenesulfenic formed when garlic is cut or crushed and to demonstrate that this sulfenic acid has a lifetime of less than one second.[8] The pharmacological activity of certain drugs, such as omeprazole, esomeprazole, ticlopidine, clopidogrel, and prasugrel is proposed to involve sulfenic acid intermediates.[9] Oxidation of cysteine residues in protein to the corresponding protein sulfenic acids is suggested to be important in redox-mediated signal transduction.[10][11]
Sulfenic acid forms part of the series of chemical reactions that occur when cutting onions. The lachrymal glands are irritated by the end product of the reactions, syn-Propanethial-S-oxide, causing tears.[12]
Organic and inorganic chemistry

Sulfoxides can undergo thermal elimination via an Ei mechanism to yield vinyl alkenes and sulfenic acids:[13][14]
Compounds which react in this manner are used as polymer stabilizers where they protects against long term heat ageing,[15] structures based on thiodipropionate esters are popular.[16]
Sulfenate-based ligands are found at the active site of the nitrile hydratases. The S=O group is proposed as the nucleophile that attacks the nitrile.[17]
Other sulfenyl compounds

The prefix sulfenyl in organic nomenclature denotes the RS group (R ≠ H). One example is methanesulfenyl chloride, CH3SCl.[18]
Sulfenate esters have the formula RSOR′. They arise by the reaction of sulfenyl chlorides on alcohols.[19] Sulfenate esters are intermediates in the Mislow-Evans rearrangement of allyl sulfoxides.[13] Sulfenamides have the formula RSNR′2.
References
- ^ Penn RE, Block E, Revelle LK (1978). "Methanesulfenic Acid". Journal of the American Chemical Society. 100 (11): 3622–3624. doi:10.1021/ja00479a068.
- ^ Goto K, Holler M, Okazaki R (1997). "Synthesis, Structure, and Reactions of a Sulfenic Acid Bearing a Novel Bowl-Type Substituent: The First Synthesis of a Stable Sulfenic Acid by Direct Oxidation of a Thiol". Journal of the American Chemical Society. 119 (6): 1460–1461. doi:10.1021/ja962994s.
- ^ Ishii A, Komiya K, Nakayama J (1996). "Synthesis of a Stable Sulfenic Acid by Oxidation of a Sterically Hindered Thiol (Thiophenetriptycene-8-thiol)1 and Its Characterization". Journal of the American Chemical Society. 118 (50): 12836–12837. doi:10.1021/ja962995k.
- ^ McGrath AJ, Garrett GE, Valgimigli L, Pratt DA (2010). "The redox chemistry of sulfenic acids". Journal of the American Chemical Society. 132 (47): 16759–16761. doi:10.1021/ja1083046. PMID 21049943.
- ^ Rhee, Sue Goo; Kil, In Sup (2017). "Multiple Functions and Regulation of Mammalian Peroxiredoxins". Annual Review of Biochemistry. 86: 749–775. doi:10.1146/annurev-biochem-060815-014431. PMID 28226215.
- ^ Block, E. (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 978-0-85404-190-9.
- ^ Vaidya V, Ingold KU, Pratt DA (2009). "Garlic: Source of the Ultimate Antioxidants – Sulfenic Acids". Angewandte Chemie International Edition. 48 (1): 157–60. doi:10.1002/anie.200804560. PMID 19040240.
- ^ Block E, Dane AJ, Thomas S, Cody RB (2010). "Applications of Direct Analysis in Real Time–Mass Spectrometry (DART-MS) in Allium Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl Trisulfane S-Oxide and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums". Journal of Agricultural and Food Chemistry. 58 (8): 4617–4625. doi:10.1021/jf1000106. PMID 20225897.
- ^ Mansuy D, Dansette PM (2011). "Sulfenic acids as reactive intermediates in xenobiotic metabolism". Archives of Biochemistry and Biophysics. 507 (1): 174–185. doi:10.1016/j.abb.2010.09.015. PMID 20869346.
- ^ Kettenhofen, NJ, Wood, MJ (2010). "Formation, Reactivity, and Detection of Protein Sulfenic Acids". Chem. Res. Toxicol. 23 (11): 1633–1646. doi:10.1021/tx100237w. PMC 2990351. PMID 20845928.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gupta, Vinayak; Kate S. Carroll (February 2014). "Sulfenic acid chemistry, detection and cellular lifetime". Biochimica et Biophysica Acta (BBA) - General Subjects. 1840 (2): 847–875. doi:10.1016/j.bbagen.2013.05.040. PMC 4184475. PMID 23748139.
- ^ "Why does chopping an onion make you cry?". Everyday Mysteries. The Library of Congress. Retrieved 1 April 2019.
- ^ a b Braverman, S., "Rearrangements involving sulfenic acids and their derivatives," in Sulfenic Acids and Derivatives, 1990, John Wiley & Sons. doi:10.1002/9780470772287.ch8
- ^ Michael Carrasco, Robert J. Jones, Scott Kamel, H. Rapoport, Thien Truong (1992). "N-(Benzyloxycarbonyl)-L-Vinylglycine Methyl Ester". Organic Syntheses. 70: 29. doi:10.15227/orgsyn.070.0029.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kröhnke, C. (2016). "Polymer Stabilization". Reference Module in Materials Science and Materials Engineering. doi:10.1016/B978-0-12-803581-8.01487-9. ISBN 978-0-12-803581-8.
- ^ Armstrong, C.; Plant, M.A.; Scott, G. (February 1975). "Mechanisms of antioxidant action: the nature of the redox behaviour of thiodipropionate esters in polypropylene". European Polymer Journal. 11 (2): 161–167. doi:10.1016/0014-3057(75)90141-X.
- ^ Harrop, Todd C.; Mascharak, Pradip K. (2004). "Fe(III) and Co(III) Centers with Carboxamido Nitrogen and Modified Sulfur Coordination: Lessons Learned from Nitrile Hydratase". Accounts of Chemical Research. 37 (4): 253–260. doi:10.1021/ar0301532. PMID 15096062.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "sulfenyl groups". doi:10.1351/goldbook.S06098
- ^ Petrovic, Goran; Saicic, Radomir N.; Cekovic, Zivorad (2005). "Phenylsulfenylation of Nonactivated Carbon Atom by Photolysiis of Alkyl Benzenesulfenated: Preparation of 2-Phenylthio-5-heptanol". Organic Syntheses. 81: 244. doi:10.15227/orgsyn.081.0244.

