| |

| |

| |
| Names | |
|---|---|
| IUPAC names
Selenous acid
Selenic(IV) acid | |
| Other names
Selenious acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.029.067 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3283 2630 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H2SeO3 | |
| Molar mass | 128.984 g·mol−1 |
| Appearance | white hygroscopic crystals |
| Density | 3.0 g/cm3 |
| Melting point | decomposes at 70 °C |
| very soluble | |
| Solubility | soluble in ethanol |
| Acidity (pKa) | pKa1 = 2.46 pKa2 = 7.3[2] |
| Conjugate base | Hydrogenselenite |
| −45.4·10−6 cm3/mol | |
| Pharmacology | |
| Intravenous infusion | |
| Legal status |
|
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P314, P321, P330, P391, P403+P233, P405, P501 | |
| Related compounds | |
Other anions
|
Selenic acid Hydrogen selenide |
Other cations
|
Sodium selenite |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| License data | |
| Identifiers | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.029.067 |
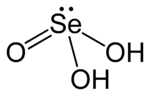
Selenous acid (or selenious acid) is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by O=Se(OH)2. It is the principal oxoacid of selenium; the other being selenic acid.
YouTube Encyclopedic
-
1/5Views:1 001 0245848 4221 2701 591
-
Naming Acids Introduction
-
Synthesis of Selenic Acid and Playing With Lanthanides
-
How to Write the Formula for Sulfurous Acid
-
Melting Selenium pellets into Ingots
-
Selenium Chemistry: SeO2 in H2O (H2SeO3)
Transcription
Formation and properties
Selenous acid is analogous to sulfurous acid, but it is more readily isolated. Selenous acid is easily formed upon the addition of selenium dioxide to water. As a crystalline solid, the compound can be seen as pyramidal molecules that are interconnected with hydrogen bonds. In solution it is a diprotic acid:[3]
- H2SeO3 ⇌ H+ + HSeO−3 (pKa = 2.62)
- HSeO−3 ⇌ H+ + SeO2−3 (pKa = 8.32)
It is moderately oxidizing in nature, but kinetically slow. In 1 M H+:
- H2SeO3 + 4 H+ + 4 e− ⇌ Se + 3 H2O (E
o= +0.74 V)
In 1 M OH−:
- SeO2−3 + 4 e− + 3 H2O ⇌ Se + 6 OH− (E
o= −0.37 V)
Selenous acid is hygroscopic.[4][5]
Uses
The major use is in protecting and changing the color of steel, especially steel parts on firearms.[6] The so-called cold-bluing process uses selenous acid, copper(II) nitrate, and nitric acid to change the color of the steel from silver-grey to blue-grey or black. Alternative procedures use copper sulfate and phosphoric acid instead. This process deposits a coating of copper selenide and is fundamentally different from other bluing processes which generate black iron oxide. Some older razor blades were also made of blued steel.[6]
Another use for selenious acid is the chemical darkening and patination of copper, brass and bronze, producing a rich dark brown color that can be further enhanced with mechanical abrasion.[citation needed]
It is used in organic synthesis as an oxidizing agent for the synthesis of 1,2-dicarbonyl compounds, e.g. in laboratory preparation of glyoxal (ethane-1,2-dione) from acetaldehyde.[7]
Selenious acid is a key component of the Mecke reagent used for drug checking.[8][9]
Medical
Selenous acid can supply the trace element indicated in people as a source of selenium.[10][11]
Health effects
Like many selenium compounds, selenous acid is highly toxic in excessive quantities, and ingestion of any significant quantity of selenous acid is usually fatal, however it is an approved dietary source in proper amounts. Symptoms of selenium poisoning can occur several hours after exposure, and may include stupor, nausea, severe hypotension and death.
References
- ^ Lide DR (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–81. ISBN 0-8493-0594-2.
- ^ Ka and pKa for Polyprotic Acids. ucdsb.on.ca
- ^ Holleman AF, Wiberg E (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Lide DR, ed. (1995). CRC Handbook of Chemistry and Physics (76th ed.). Boca Raton, FL: CRC Press Inc. pp. 4–82.
- ^ "Selenious acid". PubChem. Retrieved 2020-01-17.
- ^ a b Scarlato EA, Higa J (28 June 1990). "SELENIUM (PIM483)". Retrieved 29 December 2010.
- ^ "Glyoxal Bisulfite", Organic Syntheses, Collected Volume 3, p.438 (1955).
- ^ "Colour Test Reagents-Kits for Preliminary Identification of Drugs of Abuse" (PDF). National Institute of Justice. 2000-07-01. Retrieved 2012-01-26.
- ^ "Material Safety Data Sheet - Product Name: Reagent for Special Opiates" (PDF). Sirchie Finger Print Laboratories, Inc. May 12, 2006. Archived from the original (PDF) on October 18, 2006.
- ^ "Selenious acid injection, solution". DailyMed. 1 May 2020. Retrieved 22 October 2020.
- ^ "Drug Approval Package: Selenious Acid Injection". U.S. Food and Drug Administration (FDA). Retrieved 22 October 2020.
External links
- "Selenious acid". Drug Information Portal. U.S. National Library of Medicine.
