| |
| Names | |
|---|---|
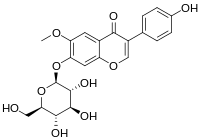
| IUPAC name
7-(β-D-Glucopyranosyloxy)-4′-hydroxy-6-methoxyisoflavone
| |
| Systematic IUPAC name
3-(4-Hydroxyphenyl)-6-methoxy-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Glycitein 7-O-glucoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H22O10 | |
| Molar mass | 446.408 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glycitin (glycitein 7-O-glucoside) is an isoflavone found in soy, and remains to various degrees in soy products like tofu, soymilk[1] and soy sauce.[2] Although glycitin has its own health associated properties (below), it can be transformed to glycitein by human intestinal flora by the action of beta-glucosidases.[3]
Properties
Some interesting effects of glycitin include human dermal fibroblast cell proliferation and migration via TGF‐β signaling, glycitin treatment produces anti-photoaging effects such as collagen type I and collagen type III increase at both the mRNA and protein levels. Other noted effects decreased elastase, and decreased β‐galactosidase activation.[4] In conjunction with 4′,6,7-trimethoxyisoflavone (TMF), an isoflavone that promotes fibroblast migration but not proliferation, wound healing and anti-scarring activity (reorganization and wound fibrosis inhibition) were significantly and synergistically boosted in both in vivo mice and in vitro.[5]
References
- ^ Hsiao, Yu-Hsuan; Yu, Chia-Jung; Li, Wen-Tai; Hsieh, Jung-Feng (2015). "Coagulation of β-conglycinin, glycinin and isoflavones induced by calcium chloride in soymilk". Scientific Reports. 5: 13018. Bibcode:2015NatSR...513018H. doi:10.1038/srep13018. PMC 4542527. PMID 26260443.
- ^ "Health Benefits of Naturally Brewed Soy Sauce". 2018-04-04.
- ^ "Human Metabolome Database: Showing metabocard for Glycitin (HMDB0002219)".
- ^ Kim, Young Mee; Huh, Jung Sik; Lim, Yoongho; Cho, Moonjae (2015). "Soy Isoflavone Glycitin (4'-Hydroxy-6-Methoxyisoflavone-7-D-Glucoside) Promotes Human Dermal Fibroblast Cell Proliferation and Migration via TGF-β Signaling". Phytotherapy Research. 29 (5): 757–769. doi:10.1002/ptr.5313. PMID 25758427. S2CID 206430410.
- ^ Kim, Joungmok; Yang, Goowon; Kim, Yeji; Kim, Jin; Ha, Joohun (2016). "AMPK activators: Mechanisms of action and physiological activities". Experimental & Molecular Medicine. 48 (4): e224. doi:10.1038/emm.2016.16. PMC 4855276. PMID 27034026.

