 | |
| Clinical data | |
|---|---|
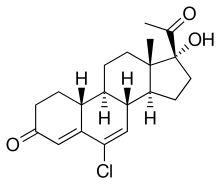
| Other names | 6-Chloro-17α-hydroxy-19-norpregna-4,6-dione |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H25ClO3 |
| Molar mass | 348.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Amadinone (INN), also known as 19-norchlormadinone, is a steroidal progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups that was synthesized and characterized in 1968 but was never marketed.[1][2] It has antigonadotropic properties, and for this reason, is a functional antiandrogen.[3][4] An acetate ester, amadinone acetate, also exists, but similarly was never marketed.[1]
See also
References
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 35–. ISBN 978-1-4757-2085-3.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 206–. ISBN 978-0-8155-1856-3.
- ^ Hughes A, Hasan SH, Oertel GW, Voss HE, Bahner F, Neumann G, Steinbeck H, Gräf KJ, Brotherton J, Horn HJ, Wagner RK (27 November 2013). Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer Science & Business Media. pp. 495–. ISBN 978-3-642-80859-3.
- ^ Kent JR, Hill M, Huix FJ, Segre EJ (1972). "Seminal acid phosphatase content in the clinical bioassay of androgens and antiandrogens". Clinical Pharmacology and Therapeutics. 13 (2): 205–11. doi:10.1002/cpt1972132205. PMID 5017374. S2CID 40886901.
