There are several Akabori amino acid reactions, which are named after Shirō Akabori (Japanese: 赤堀 四郎) (1900–1992), a Japanese chemist.
In the first reaction, an α-amino acid is oxidised and undergoes decarboxylation to give an aldehyde at the former α position by heating with oxygen in the presence of a reducing sugar.[1][2][3] This reaction is useful for preparing dichlorophthalimido derivatives[how?] of peptides for mass spectral analysis.[4]
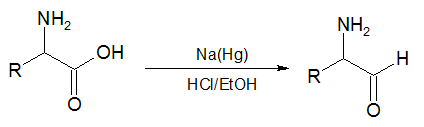
In the second reaction, an α-amino acid, or an ester of it, is reduced by sodium amalgam and ethanolic HCl to give an α-amino aldehyde.[5][6] This process is conceptually similar to the Bouveault–Blanc reduction[7][8][9] except that it stops at the aldehyde stage rather than reducing the ester all the way to two alcohols.
YouTube Encyclopedic
-
1/3Views:26 8242 44123 103
-
Reactions of Amino Acids
-
PHENYLPROPANOLAMINE SYNTHESIS
-
Determining the N-terminal Residue
Transcription
See also
References
- ^ S. Akabori (1931). "Amino Acids and Their Derivatives (I) Oxidative Decomposition of α-Amino Acids with Sugars. (I)". Nippon Kagaku Kaishi. 52: 606–610. doi:10.1246/nikkashi1921.52.606.
- ^ S. Akabori (1931). "Oxidative Degradation of α-Amino Acids with Sugars (II). On the Mechanisms of the Reaction. (Amino Acids and their Derivatives. II.)". Nippon Kagaku Kaishi. 52 (12): 839–843. doi:10.1246/nikkashi1921.52.839.
- ^ S. Akabori (1933). "Oxydativer Abbau von α-Amino-säuren durch Zucker". Chem. Ber. (in German). 66 (2): 143. doi:10.1002/cber.19330660213.
- ^ "Akabori Amino Acid Reaction". Comprehensive Organic Name Reactions and Reagents. 8: 29–32. 2010. doi:10.1002/9780470638859.conrr008. ISBN 9780470638859.
- ^ A. Lawson, H.V. Motley (1955). "2-Mercaptoglyoxalines. Part IX. The preparation of 1 : 5-disubstituted 2-mercaptoglyoxalines from α-amino-acids". J. Chem. Soc.: 1695–1698. doi:10.1039/jr9550001695.
- ^ A. Lawson (1956). "63. The reaction of cyanamide with α-amino-acetals and α-amino-aldehydes". J. Chem. Soc.: 307–310. doi:10.1039/jr9560000307.
- ^ Bouveault, Louis; Blanc, Gustave Louis (1903). "Préparation des alcools primaires au moyen des acides correspondants" [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. (in French). 136: 1676–1678.
- ^ Bouveault, Louis; Blanc, Gustave Louis (1903). "Préparation des alcools primaires au moyen des acides correspondants" [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. (in French). 137: 60–62.
- ^ Bouveault, Louis; Blanc, Gustave Louis (1904). "Transformation des acides monobasiques saturés dans les alcools primaires correspondants" [Transforming saturated monobasic acids into the corresponding primary alcohols]. Bull. Soc. Chim. Fr. (in French). 31: 666–672.