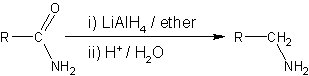Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.[1][2]
YouTube Encyclopedic
-
1/3Views:1 834254 39726 254
-
Reduction of amides
-
(L-7) Preparation of R-NH2 (Amines) By Reduction of Amide (R-Co-NH2) || NEET JEE || By A. Arora
-
Mechanisms of Amide Reduction by LiAlH4
Transcription
Catalytic hydrogenation
Catalytic hydrogenation can be used to reduce amides to amines; however, the process often requires high hydrogenation pressures and reaction temperatures to be effective (i.e. often requiring pressures above 197 atm and temperatures exceeding 200 °C).[1] Selective catalysts for the reaction include copper chromite, rhenium trioxide and rhenium(VII) oxide or bimetallic catalyst.[3][4][5]
Amines from other hydride sources
Reducing agents able to effect this reaction include metal hydrides such as lithium aluminium hydride,[6][7][8][9][10] or lithium borohydride in mixed solvents of tetrahydrofuran and methanol.[11]
Iron catalysis by triiron dodecacarbonyl in combination with polymethylhydrosiloxane has been reported.[12]
Noncatalytic routes to aldehydes
Some amides can be reduced to aldehydes in the Sonn-Müller method, but most routes to aldehydes involve a well-chosen organometallic reductant.
Lithium aluminum hydride reduces an excess of N,N-disubstituted amides to an aldehyde:[citation needed]
- R(CO)NRR' + LiAlH4 → RCHO + HNRR'
With further reduction the alcohol is obtained.
Schwartz's reagent reduces amides to aldehydes,[13] and so does hydrosilylation with a suitable catalyst.
References
- ^ a b Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 406–411. ISBN 9780471396987.
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ Mitsudome, Takato; Miyagawa, Kazuya; Maeno, Zen; Mizugaki, Tomoo; Jitsukawa, Koichiro; Yamasaki, Jun; Kitagawa, Yasutaka; Kaneda, Kiyotomi (2017-08-01). "Mild Hydrogenation of Amides to Amines over a Platinum-Vanadium Bimetallic Catalyst". Angewandte Chemie International Edition. 56 (32): 9381–9385. doi:10.1002/anie.201704199. PMID 28649715.
- ^ Zhang, Yue; Zhang, Fan; Li, Lin; Liu, Fei; Wang, Aiqin (2022-10-07). "Highly Chemoselective Reduction of Amides to Amines over a Ruthenium‐Molybdenum Bimetallic Catalyst". ChemistrySelect. 7 (37). doi:10.1002/slct.202203030. ISSN 2365-6549. S2CID 252725710.
- ^ Pennetier, Alex; Hernandez, Willinton Y.; Kusema, Bright T.; Streiff, Stéphane (2021-08-25). "Efficient hydrogenation of aliphatic amides to amines over vanadium-modified rhodium supported catalyst". Applied Catalysis A: General. 624: 118301. doi:10.1016/j.apcata.2021.118301. ISSN 0926-860X. S2CID 238850541.
- ^ Cope, Arthur C.; Ciganek, Engelbert (1959). "N,N-Dimethylcyclohexylmethylamine". Organic Syntheses. 39: 19. doi:10.15227/orgsyn.039.0019.
- ^ Wilson, C. V.; Stenberg, J. F. (1956). "Laurylmethylamine". Organic Syntheses. 36: 48. doi:10.15227/orgsyn.036.0048.
- ^ Moffett, Robert Bruce (1953). "2,2-Dimethylpyrrolidine". Organic Syntheses. 33: 32. doi:10.15227/orgsyn.033.0032.
- ^ Park, Chung Ho; Simmons, Howard E. (1974). "Macrocyclic Diimines: 1,10-Diazacylooctadecane". Organic Syntheses. 54: 88. doi:10.15227/orgsyn.054.0088.
- ^ Seebach, Dieter; Kalinowski, Hans-Otto; Langer, Werner; Crass, Gerhard; Wilka, Eva-Maria (1983). "Chiral Media for Asymmetric Solvent Inductions". Organic Syntheses. 61: 24. doi:10.15227/orgsyn.061.0024.
- ^ Ookawa, Atsuhiro; Soai, Kenso (1986). "Mixed solvents containing methanol as useful reaction media for unique chemoselective reductions within lithium borohydride". The Journal of Organic Chemistry. 51 (21): 4000–4005. doi:10.1021/jo00371a017.
- ^ Zhou, S.; Junge, K.; Addis, D.; Das, S.; Beller, M. (2009). "A Convenient and General Iron-Catalyzed Reduction of Amides to Amines". Angewandte Chemie International Edition in English. 48 (50): 9507–9510. doi:10.1002/anie.200904677. PMID 19784999.
- ^ Leighty, M. W.; Spletstoser, J. T.; Georg, Gunda I. (2011). "Mild Conversion of Tertiary Amides to Aldehydes Using Cp2ZrHCl (Schwartz's Reagent)". Org. Synth. 88: 427–437. doi:10.1002/0471264229.os088.39. ISBN 978-0471264224.