| Passerini reaction | |
|---|---|
| Named after | Mario Passerini |
| Reaction type | Carbon-carbon bond forming reaction |
| Identifiers | |
| Organic Chemistry Portal | passerini-reaction |
| RSC ontology ID | RXNO:0000244 |
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde (or ketone), and a carboxylic acid to form a α-acyloxy amide.[1][2][3][4][5] This addition reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy.[6][7] It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or deep eutectic solvents.[7] It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry.[6][8][9] As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.[6][10][11][12]
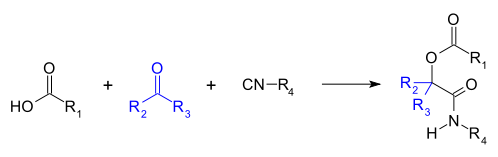
YouTube Encyclopedic
-
1/5Views:11 34515 5721 4761 4421 671
-
Passerini Reaction
-
Ugi Reaction
-
Passerini rexn
-
Passerini Reaction
-
Passerini Reaction by Ted
Transcription
Mechanism
The Passerini reaction has been hypothesized to occur through two mechanistic pathways.[10][7][11] The reaction pathways are dependent on the solvent used.
Concerted mechanism
A concerted mechanism, seen in SN2 and Diels−Alder reactions, is theorized to occur when the Passerini reagents are present at high concentration in aprotic solvents.[10]

This mechanism involves a trimolecular reaction between the isocyanide, carboxylic acid, and carbonyl in a sequence of nucleophilic additions. The reaction proceeds first through an imidate intermediate and then undergoes Mumm rearrangement to afford the Passerini product.[13][14]
As the Mumm rearrangement requires a second carboxylic acid molecule, this mechanism classifies the Passerini reaction as an organocatalytic reaction.[14][15]
Ionic mechanism

In polar solvents, such as methanol or water, the carbonyl is protonated before nucleophilic addition of the isocyanide, affording a nitrilium ion intermediate. This is followed by the addition of a carboxylate, acyl group transfer and proton transfer respectively to give the desired Passerini product.[11][7]
Reaction control
Molecular weights of polymers synthesized through the Passerini can be controlled through stoichiometric means.[16] For example, polymer chain length and weight can adjusted through isocyanide stoichiometry, and polymer geometry can be influenced through starting reagents.[16][17] To facilitate the Passerini reaction between bulky, sterically hindered reagents, a vortex fluidic device can be used to induce high shear conditions. These conditions emulate the effects of high temperature and pressure, allowing the Passerini reaction to proceed fairly quickly.[18] The Passerini reaction can also exhibit enantioselectivity. Addition of tert-butyl isocyanide to a wide variety of aldehydes (aromatic, heteroaromatic, olefinic, acetylenic, aliphatic) is achieved using a catalytic system of tetrachloride and a chiral bisphosphoramide which provides good yield and good enantioselectivities.[19] For other types of isocyanides, rate of addition of isocyanide to reaction mixture dictates good yields and high selectivities.[19]
Applications
Apart from forming α-acyloxy amide products, the Passerini reaction can be used to form heterocycles, polymers, amino acids, and medicinal products.
Heterocycles

The original Passerini reaction produces acyclic depsipeptides which are labile in physiological conditions. To increase product stability for medicinal use, post-Passerini cyclization reactions have been used to afford heterocycles such as β-lactams, butenolides, and isocoumarins.[16] To enable these cyclizations, reagents are pre-functionalized with reactive groups (ex. halogens, azides, etc.) and used in tandem with other reactions (ex. Passerini-Knoevenagel, Passerini-Dieckmann) to afford heterocyclic products.[16] Compounds like three membered oxirane and aziridine derivatives, four-membered b-lactams, and five-membered tetrasubstituted 4,5-dihydropyrazoles have been produced through this reaction.[12]
Polymers

This reaction has also been used for polymerization, monomer formation, and post-polymerization modification.[20][21][22][17][23] The Passerini reaction has also been used to form sequence-defined polymers.[24] Bifunctional substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization.[10][11][8] As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties.[20] Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component dendrimers and three-armed star branched mesogen core molecules.[12]
Amino acids and pharmaceuticals
Passerini reaction has been employed for the formation of structures like α-amino acids, α-hydroxy-β-amino acids, α-ketoamides, β-ketoamides, α-hydroxyketones and α-aminoxyamides.[12] The Passerini reaction has synthesized α-Acyloxy carboxamides that have demonstrated activity as anti-cancer medications along with functionalized [C60]-fullerenes used in medicinal and plant chemistry.[12][25] This reaction has also been used as a synthetic step in the total synthesis of commercially available pharmaceuticals such as telaprevir (VX-950), an antiviral sold by Vertex Pharmaceuticals and Johnson & Johnson.[12]

See also
References
- ^ Passerini, M.; Simone, L. Gazz. Chim. Ital. 1921, 51, 126–29.
- ^ Passerini, M.; Ragni, G. Gazz. Chim. Ital. 1931, 61, 964–69.
- ^ Banfi, L.; Riva, R. (2005). The Passerini Reaction. Vol. 65. pp. 1–140. doi:10.1002/0471264180.or065.01. ISBN 978-0471264187.
{{cite book}}:|journal=ignored (help). - ^ Kazemizadeh, A.R.; Ramazani, A. (2012). "Synthetic applications of Passerini reaction". Curr. Org. Chem. 16 (4): 418–450. doi:10.2174/138527212799499868.
- ^ Banfi, L.; Basso, A.; Lambruschini, C.; Moni, L.; Riva, R. (2021). "The 100 facets of the Passerini reaction". Chem. Sci. 12 (47): 15445–15472. doi:10.1039/D1SC03810A. PMC 8654045. PMID 35003575.
- ^ a b c Tuten, Bryan T.; Bui, Aaron H.; Wiedbrauk, Sandra; Truong, Vinh X.; Raston, Colin L.; Barner-Kowollik, Christopher (19 August 2021). "Four component Passerini polymerization of bulky monomers under high shear flow". Chemical Communications. 57 (67): 8328–8331. doi:10.1039/D1CC02984C. ISSN 1364-548X. PMID 34323263. S2CID 236498755.
- ^ a b c d Antenucci, Achille; Marra, Francesco; Dughera, Stefano (2021). "Silica gel-immobilised chiral 1, 2-benzenedisulfonimide: a Brønsted acid heterogeneous catalyst for enantioselective multicomponent Passerini reaction". RSC Advances. 11 (42): 26083–26092. Bibcode:2021RSCAd..1126083A. doi:10.1039/D1RA05297G. PMC 9037113. PMID 35479468.
- ^ a b Abbasi, Elham; Aval, Sedigheh Fekri; Akbarzadeh, Abolfazl; Milani, Morteza; Nasrabadi, Hamid Tayefi; Joo, Sang Woo; Hanifehpour, Younes; Nejati-Koshki, Kazem; Pashaei-Asl, Roghiyeh (21 May 2014). "Dendrimers: synthesis, applications, and properties". Nanoscale Research Letters. 9 (1): 247. Bibcode:2014NRL.....9..247A. doi:10.1186/1556-276X-9-247. ISSN 1556-276X. PMC 4074873. PMID 24994950.
- ^ Dömling, A.; Ugi, I. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168–3210. (Review)
- ^ a b c d The Passirini Reaction L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 ISBN 0-471-68260-8
- ^ a b c d Taran, Jafar; Ramazani, Ali; Joo, Sang Woo; Ślepokura, Katarzyna; Lis, Tadeusz (2014). "Synthesis of Novel a-(Acyloxy)-a-(quinolin-4-yl)acetamides by a ThreeComponent Reaction between an Isocyanide, Quinoline-4-carbaldehyde, and Arenecarboxylic Acids". Helvetica Chimica Acta. 97: 1088–1096. doi:10.1002/hlca.201300378.
- ^ a b c d e f Wahby, Yasmin; Abdel-Hamid, Hamida; Ayoup, Mohammed Salah (2022). "Two decades of recent advances of Passerini reactions: synthetic and potential pharmaceutical applications". New Journal of Chemistry. 46 (4): 1445–1468. doi:10.1039/D1NJ03832J. ISSN 1144-0546. S2CID 245431805.
- ^ Li, Jie Jack (2021), Li, Jie Jack (ed.), "Passerini Reaction", Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications, Cham: Springer International Publishing, pp. 424–426, doi:10.1007/978-3-030-50865-4_115, ISBN 978-3-030-50865-4, retrieved 24 October 2022
- ^ a b Ramozzi, Romain; Morokuma, Keiji (5 June 2015). "Revisiting the Passerini Reaction Mechanism: Existence of the Nitrilium, Organocatalysis of Its Formation, and Solvent Effect". The Journal of Organic Chemistry. 80 (11): 5652–5657. doi:10.1021/acs.joc.5b00594. ISSN 0022-3263. PMID 25974627.
- ^ Marcelli, Tommaso; Olimpieri, Francesca; Volonterio, Alessandro (29 June 2011). "Domino synthesis of 1,3,5-trisubstituted hydantoins: a DFT study". Organic & Biomolecular Chemistry. 9 (14): 5156–5161. doi:10.1039/C1OB05242J. ISSN 1477-0539. PMID 21643563.
- ^ a b c d Oelmann, S.; Solleder, S. C.; Meier, M. a. R. (1 March 2016). "Controlling molecular weight and polymer architecture during the Passerini three component step-growth polymerization". Polymer Chemistry. 7 (10): 1857–1860. doi:10.1039/C5PY02030A. ISSN 1759-9962.
- ^ a b Rudick, Jonathan G. (October 2013). "Innovative macromolecular syntheses via isocyanide multicomponent reactions". Journal of Polymer Science Part A: Polymer Chemistry. 51 (19): 3985–3991. Bibcode:2013JPoSA..51.3985R. doi:10.1002/pola.26808.
- ^ Tuten, Bryan T.; Bui, Aaron H.; Wiedbrauk, Sandra; Truong, Vinh X.; Raston, Colin L.; Barner-Kowollik, Christopher (2021). "Four component Passerini polymerization of bulky monomers under high shear flow". Chemical Communications. 57: 8328–8331. doi:10.1039/D1CC02984C.
- ^ a b Denmark, Scott E.; Fan, Yu (1 November 2005). "Catalytic, Enantioselective α-Additions of Isocyanides: Lewis Base Catalyzed Passerini-Type Reactions". The Journal of Organic Chemistry. 70 (24): 9667–9676. doi:10.1021/jo050549m. ISSN 0022-3263. PMID 16292793.
- ^ a b Kreye, Oliver; Tóth, Tommy; Meier, Michael A. R. (16 February 2011). "Introducing Multicomponent Reactions to Polymer Science: Passerini Reactions of Renewable Monomers". Journal of the American Chemical Society. 133 (6): 1790–1792. doi:10.1021/ja1113003. ISSN 0002-7863. PMID 21265532.
- ^ Li, Lei; Lv, An; Deng, Xin-Xing; Du, Fu-Sheng; Li, Zi-Chen (28 August 2013). "Facile synthesis of photo-cleavable polymers via Passerini reaction". Chemical Communications. 49 (76): 8549–8551. doi:10.1039/C3CC44557G. ISSN 1364-548X. PMID 23945608.
- ^ Sehlinger, Ansgar; Kreye, Oliver; Meier, Michael A. R. (13 August 2013). "Tunable Polymers Obtained from Passerini Multicomponent Reaction Derived Acrylate Monomers". Macromolecules. 46 (15): 6031–6037. Bibcode:2013MaMol..46.6031S. doi:10.1021/ma401125j. ISSN 0024-9297.
- ^ Travanut, Alessandra; Monteiro, Patrícia F.; Oelmann, Stefan; Howdle, Steven M.; Grabowska, Anna M.; Clarke, Philip A.; Ritchie, Alison A.; Meier, Michael A. R.; Alexander, Cameron (March 2021). "Synthesis of Passerini‐3CR Polymers and Assembly into Cytocompatible Polymersomes". Macromolecular Rapid Communications. 42 (6): 2000321. doi:10.1002/marc.202000321. ISSN 1022-1336. PMID 33249682. S2CID 225447799.
- ^ Solleder, Susanne C.; Meier, Michael A. R. (13 January 2014). "Sequence Control in Polymer Chemistry through the Passerini Three-Component Reaction". Angewandte Chemie International Edition. 53 (3): 711–714. doi:10.1002/anie.201308960. PMID 24307280.
- ^ Ravanello, Bruno B.; Seixas, Nalin; Rodrigues, Oscar E. D.; da Silva, Rafael S.; Villetti, Marcos A.; Frolov, Andrej; Rivera, Daniel G.; Westermann, Bernhard (11 July 2018). "Diversity Driven Decoration and Ligation of Fullerene by Ugi and Passerini Multicomponent Reactions". Chemistry – A European Journal. 24 (39): 9788–9793. doi:10.1002/chem.201802414. PMID 29882608. S2CID 46969435.
