| |
| Names | |
|---|---|
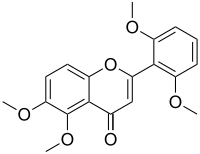
| IUPAC name
2′,5,6,6′-Tetramethoxyflavone
| |
| Systematic IUPAC name
2-(2,6-Dimethoxyphenyl)-5,6-dimethoxy-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H18O6 | |
| Molar mass | 342.347 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zapotin is a natural chemical compound, classified as a flavone, isolated from White sapote (Casimiroa edulis).[1]
Several recent in vitro studies have shown that zapotin has potential anti-carcinogenic effects against isolated colon cancer cells.[2][3]
References
- ^ Sondheimer F (1960). "Constituents of Casimiroa edulis Llave et Lex.—VI 2′,5,6-Trimethoxyflavone, 2′,5,6,7-tetramethoxyflavone (zapotin) and 5-hydroxy-2′,6,7-trimethoxyflavone (zapotinin)". Tetrahedron. 9 (3–4): 139–144. doi:10.1016/0040-4020(60)80001-4.
- ^ Murillo G, Hirschelman WH, Ito A, Moriarty RM, Kinghorn AD, Pezzuto JM, et al. (2007). "Zapotin, a phytochemical present in a Mexican fruit, prevents colon carcinogenesis". Nutrition and Cancer. 57 (1): 28–37. doi:10.1080/01635580701268097. PMID 17516860. S2CID 20080099.
- ^ Maiti A, Cuendet M, Kondratyuk T, Croy VL, Pezzuto JM, Cushman M (Jan 2007). "Synthesis and cancer chemopreventive activity of zapotin, a natural product from Casimiroa edulis". Journal of Medicinal Chemistry. 50 (2). American Chemical Society: 350–5. doi:10.1021/jm060915+. PMC 2523270. PMID 17228877.
