 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.438 |
| Chemical and physical data | |
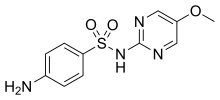
| Formula | C11H12N4O3S |
| Molar mass | 280.30 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sulfametoxydiazine (INN) or sulfamethoxydiazine (USAN: sulfameter) is a long-acting sulfonamide antibacterial.[1] It is used as a leprostatic agent and in the treatment of urinary tract infections.[2]
Sulfamethoxydiazine is also used to treat and prevent diseases in animals. Because of its relatively long persistence, sulfamethoxydiazine residue can be detected in meat, dairy, and eggs, and is considered hazardous to human health. The United States and Japan both prohibit sulfamethoxydiazine residue in food, whereas the Codex Alimentarius Commission states that the maximum limit for sulfonamides in animal tissues is 100 μg/kg.[1]
References
- ^ a b Wu Y, Yu S, Yu F, Yan N, Qu L, Zhang H (October 2011). "Chemiluminescence enzyme immunoassay for the determination of sulfamethoxydiazine". Spectrochimica Acta Part A. 81 (1): 544–547. Bibcode:2011AcSpA..81..544W. doi:10.1016/j.saa.2011.06.047. PMID 21795101.
- ^ Burros HM, Gillenwater JY (July 1965). "Clinical Experience with Sulfamethoxydiazine* in Urinary Tract Infections". The Journal of Urology. 94 (1): 86–88. doi:10.1016/S0022-5347(17)63576-6. PMID 14319481.
