| |
| Names | |
|---|---|
| Other names
Sizofiran
| |
| Identifiers | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| Properties | |
| (C6H10O5)n | |
| Molar mass | variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
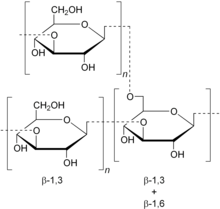
Schizophyllan (Sonifilan, SPG) is a neutral extracellular polysaccharide produced by the fungus Schizophyllum commune. Schizophyllan is a β-1,3 beta-glucan with β-1,6 branching. Schizophyllan is also known as sizofiran.
Schizophyllan has a molecular weight of 450,000 Da, and a specific rotation in water of +18-24°. A chemically analogous polysaccharide, scleroglucan, is formed by the fungus Athelia rolfsii. Both polysaccharides share the chemical structure of the backbone with curdlan. It is known for several things, including its ability to stimulate the immune system, carry metals in water, aid in delivering drugs, and use in some nanofibers.[1]
YouTube Encyclopedic
-
1/3Views:20 758163 498960
-
Schizophyllum commune in Vancouver
-
The Immune System: Innate Defenses and Adaptive Defenses
-
Microbial exopolysaccharides synthesis and metabolism| Explained| Agricultural Microbiology
Transcription
Safety
Schizophyllan, classified as a beta-glucan and extracted from the fungus Schizophyllum commune, showed no quantifiable adverse reactions through independent scientific procedures. With its classification as a beta-glucan, schizophyllan is generally recognized as safe (GRAS) by the United States FDA. While no distinction has been made specifically for schizophyllan, schizophyllan and other β-glucans have been orally administered safely in a variety of vertebrate species showing immunomodulation: mice,[2][3][4][5] canines,[6] horses,[7] and humans.[8]
More specifically, in 2012, mushroom-derived beta-glucans were deemed GRAS by direct FDA review.[9] A March 2017 further demonstrates the safety of schizophyllan in horses.[10]
Processing and efficacy
The ability for schizophyllan to produce a physiological response is directly correlated with the extraction process and subsequent processing the compound endures. This theory also coincides with that of other beta-glucans.
High doses of schizophyllan are not the primary determinant of an immunological response. Studies have validated that 10 mg (or less) of a high quality, adequately processed chemically similar beta-glucan is a sufficient dose to elicit a measurable effect on immune cells. In addition, small particle beta-glucans modified to prevent re-aggregation during digestion have the most positive effects on immune potentiation.[11]
Ninety percent of horses with active ulceration treated with ta schizophyllan-containing polysaccharide blend showed complete resolution and/or improvement in ulcerative areas, increased appetite, weight gain, and positive behavioral changes.[10]
References
- ^ Kony, David B.; Damm, Wolfgang; Stoll, Serge; Van Gunsteren, Wilfred F.; Hunenberger, Philippe H. (2007). "Explicit-Solvent Molecular Dynamics Simulations of the Polysaccharide Schizophyllan in Water". Biophysical Journal. 93 (2): 442–55. Bibcode:2007BpJ....93..442K. doi:10.1529/biophysj.106.086116. PMC 1896245. PMID 17237195.
- ^ Hotta H, Hagiwara K, Tabata K, Ito W, Homma M (Jan 1993). "Augemtnation of protective immune responses against Sendai virus infection by fungal polysaccharide schizophyllan". International Journal of Immunopharmacology. 15 (1): 55–60. doi:10.1016/0192-0561(93)90031-s. PMID 7679379.
- ^ Itoh, W (1997). "Augemtnation of protective immune responses against viral infection by oral administration of schizophyllan". Mediators of Inflammation. 6 (4): 267–269. doi:10.1080/09629359791596. PMC 2365866. PMID 18472856.
- ^ Koike, K (Dec 1976). "Protective effect of schizophyllan on Pseudomonas aeruginosa infection of mouse". Japanese Journal of Antibiotics. 29 (12): 1098–1105. PMID 137992.
- ^ Wasser, SP (1999). "Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: a modern perspective". Crit Rev Immunol. 19 (1): 65–96. doi:10.1615/critrevimmunol.v19.i1.30. PMID 9987601.
- ^ Stuyven, E (2010). "Oral administration of beta-1,3/1,6-glucan to dogs temporally changes total and antigen-specific IgA and IgM". Clin Vaccine Immunol. 17 (2): 281–285. doi:10.1128/cvi.00344-09. PMC 2815531. PMID 20032218.
- ^ Krakowski, K (1999). "The effect of nonspecific immunostimulation of pregnant mares with 1,3/1,6 glucan and levamisole on the immunoglobulins levels in colostrum, selected indices of nonspecific cellular and humoral immunity in foals in neonatal and postnatal period". Vet Immunol Immunopathol. 68 (1): 1–11. doi:10.1016/s0165-2427(99)00006-9. PMID 10231947.
- ^ Okamura, K (1989). "Adjuvant immunotherapy: two randomized controlled studies of patients with cervical cancer". Biomed Pharmacother. 43 (3): 177–181. doi:10.1016/0753-3322(89)90212-6. PMID 2528386.
- ^ "US FDA GRAS Determination - Mushroom-derived beta-glucans" (PDF). Food and Drug Administration.
- ^ a b Slovis, N (2017). "Polysaccharide Treatment Reduces Gastric Ulceration in Active Horses". Journal of Equine Veterinary Science. 50: 116–120. doi:10.1016/j.jevs.2016.11.011. S2CID 11169402.
- ^ Hunter, K (Jan 2001). "Mode of Action of B-Glucan Immunopotentiators".
{{cite journal}}: Cite journal requires|journal=(help)
External links
- Cancer Research UK - Medicinal Mushrooms: Their Therapeutic Properties and Current Medical Usage with Special Emphasis on Cancer Treatments
