| |
| |
| Names | |
|---|---|
| IUPAC name
5-Amino-3,5-dideoxy-d-glycero-d-galacto-non-2-ulosonic acid
| |
| Systematic IUPAC name
(4S,5R,6R,7S,8R)-5-Amino-4,6,7,8,9- | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Neuraminic+Acids |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H17NO8 | |
| Molar mass | 267.233 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
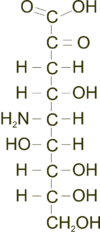
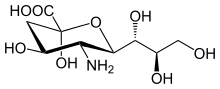
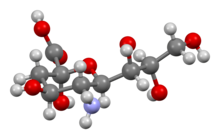
Neuraminic acid (5-amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid) is an acidic (in particular ulosonic) amino sugar with a backbone formed by nine carbon atoms.[1] Although 9-carbon sugars do not occur naturally, neuraminic acid may be regarded as a theoretical 9-carbon ketose in which the first link of the chain (the –CH2OH at position 1) is oxidised into a carboxyl group (–C(=O)OH), the hydroxyl group at position 3 is deoxidised (oxygen is removed from it), and the hydroxyl group at position 5 is substituted with an amino group (–NH2). Neuraminic acid may also be visualized as the product of an aldol-condensation of pyruvic acid and D-mannosamine (2-amino-2-deoxy-mannose).[1]
Neuraminic acid does not occur naturally, but many of its derivatives are found widely distributed in animal tissues and in bacteria, especially in glycoproteins and gangliosides. The N- or O-substituted derivatives of neuraminic acid are collectively known as sialic acids, the predominant form in mammalian cells being N-acetylneuraminic acid. The amino group bears either an acetyl or a glycolyl group. The hydroxyl substituents may vary considerably: acetyl, lactyl, methyl, sulfate and phosphate groups have been found.
The name "neuraminic acid" was introduced by German scientist E. Klenk in 1941, in reference to the brain lipids from which it was derived as a cleavage product.[2]
The IUPAC symbol used for neuraminic acid is Neu, and the residue is typically found with additional chemical modifications in biological systems. As a family, these residues are known as sialic acids. For example, N-acetylneuraminic acid, Neu5Ac, is typical in human glycoproteins.
Among their many biological functions, these structures are substrates for neuraminidase enzymes which cleave neuraminic acid residues. Human flu viruses have a neuraminidase enzyme, signified in the name "H#N#", where the H refers to an isoform of hemagglutinin and N refers to an isoform of viral neuraminidase.
YouTube Encyclopedic
-
1/5Views:7 7966421 26121 8041 852
-
Function of Sialic Acid | John Pepper
-
Neuraminic acid Meaning
-
Muramic Acid Vs Neuraminic Acid || Detailed Video....By Chiki's Biology
-
Glycosaminoglycans/Proteoglycans - Biochemistry
-
Sialic acid Meaning
Transcription
See also
References
- ^ a b Blanco, Antonio; Blanco, Gustavo (2017-01-01), Blanco, Antonio; Blanco, Gustavo (eds.), "Chapter 4 - Carbohydrates", Medical Biochemistry, Academic Press, pp. 73–97, doi:10.1016/b978-0-12-803550-4.00004-5, ISBN 978-0-12-803550-4, retrieved 2020-12-16
- ^ Klenk, E. 1941. Neuraminsäure, das Spaltprodukt eines neuen Gehirnlipoids. Zeitschr. f. Physiol. Chem. 1941; 268:50-58.
