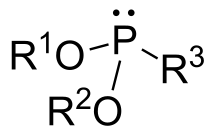
In organic chemistry, phosphonites are organophosphorus compounds with the formula P(OR)2R. They are found in some pesticides and are used as ligands.[1]
Preparation
Although they are derivatives of phosphonous acid (RP(OH)2),[2] they are not prepared from such precursors. Phosphonites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of dichlorophenylphosphine with methanol and base gives dimethyl phenylphosphonite:
- Cl2PPh + 2 CH3OH → (CH3O)2PPh + 2 HCl
Reactions
Oxidation of phosphonites gives phosphonates:
- 2 P(OR)2R + O2 → 2 OP(OR)2R
Phosphonites can function as ligands in homogeneous catalysis.[3]
References
- ^ D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam 1995. ISBN 0-444-89307-5.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "phosphonous acids". doi:10.1351/goldbook.P04565
- ^ T. V. (Babu) Rajanbabu “Phosphinite and Phosphonite Ligands” in Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis Paul C. J. Kamer and Piet W. N. M. van Leeuwen, Eds., John Wiley & Sons 2012. doi:10.1002/9781118299715.ch5
