
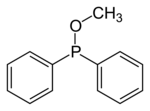
In organic chemistry, phosphinites are organophosphorus compounds with the formula P(OR)R2. They are used as ligands in homogeneous catalysis and coordination chemistry.[1]
YouTube Encyclopedic
-
1/1Views:2 739
-
Mod-09 Lec-38 Asymmetric Catalysis
Transcription
Organometallic chemistry plays a very important role in asymmetric catalysis, and in asymmetric synthesis. Look at the two chiral molecules which we see on the screen, you will realize that the two molecules are different only in the sense of rotation around the carbon, where the O H group is present. In every other way they are equivalent and so they will have similar properties. Almost all chemical properties and physical properties will be same except when these molecules interact with other chiral species. So, when you want to synthesize these molecules in a pure form either R 2 butanol or in the independently S 2 butanol you need some special techniques, and that is the topic of our lecture, today. We have a brief outline here. First we will talk about the need for making chiral molecules and the possible routes by which we can make these chiral molecules before we talk about asymmetric catalysis and organometallic chemistry. Following this, we will discuss the chiral ligand design which probably plays a very important role and then we will talk about main group asymmetric catalysis. Here, the metal atom that is playing an important role is not a transition metal, but one of the main group elements. Let us proceed, now and talk about the need for making chiral molecules. If you look at the biological activity of molecules, especially those which contain an asymmetric carbon, you soon find that in many cases these molecules have different properties, biological properties when it comes to their reaction with a body. So, here are 2 molecules naproxen which is R isomer and an S isomer. The S isomer is a drug for arthritis whereas the R isomer unfortunately is a liver poison. It carries out liver poisoning and this is a serious issue because if you give the mixture of these 2 chiral molecules to the patient, the patient will have relief from arthritis, but might die from liver poisoning. So, this is not an isolated incident, you can look at the listing here where nearly four molecules are listed. All of them have got very different reactions with the body, when the chirality is changed you go from a beneficial reaction to an extremely wrong reaction or a reaction which is adverse to the body. So, it is important that we make chiral molecules enhance pure drugs so that patient does not suffer and relief is really significant. Not only pharmaceuticals and the therapeutics, you also need to have chiral molecules to make ligands, which are chiral which we will just see presently the importance of making chiral ligands and chiral resolving agents. If you are not able to make a molecule in a chiral form, then you need to be in a position to separate the two isomers so that the pure form of the drug can be used as a drug. So, it is important to have these chiral auxiliaries and dissolving agent, we will talk about them in a minute. So, this whole area of making chiral molecule stands out to be an extremely important one. This lecture is just a preview, we are not going to be able to cover all the topics, so we will just give you peak into the important subject that we have before us today. So, let us go through the possible routes for making chiral molecules. The first one of course is a trivial one we can make the molecule as a resinic mixture and then separate or remove the unwanted isomer. Unfortunately, when we do this, the yield automatically drops to 50 percent because the resinic form both the two isomers. The two chiral forms are in equal proportions, and because we will have synthetic losses in synthetic step. Also in the purification step you will end up with a yield which is less than 50 percentage. So, this stands out be an extremely undesirable route, but nevertheless significant advances have been made in separation technology. Special mention must be made about the simulated moving bed, or counter current separation of molecules on a chromatograph. So, this type of a batch separation or a counter current separation allows for the pure form of each isomer even if you make the chiral form in an impure state, if you would be able to purify it very well using these special techniques. So, chirality by separation not only gives you wastage of the compound, you have to spend more time in order to get the pure form. So, one way is to convert the unwanted isomer, so you can think of a situation when you convert the unwanted isomer. So, suppose the R isomer, you take the S isomer and convert it into the R isomer, so this type of a conversion will allow you to separate the two isomers again. If you want to prepare more than 96 percent of the one the isomer that you want you need to get you need to carry out almost five steps. So, although this is difficult sometimes, it is useful if you just cannot make the chiral form of the molecule you try, and convert the unwanted form of the molecule to the right form. Obviously, the best way to do it is to introduce chirality in the synthesis and very often the answer to this question turns out to be this logically unacceptable answer. It is a circular form of reasoning you say how you introduce chirality in the molecule that you want to synthesize. The answer is you start with the chirally chiral molecule, which is available already. So, in a sense this is a circular reasoning you need to have a chiral molecule in order to make a chiral molecule. So, the billion dollar question is, how did chiralty come into nature into universe? The answer that is sometimes received in text books is that it is a cosmic accident, so we will leave it here because it is more of a philosophical question. So, let us proceed to the synthesis of these chiral molecules one way in which you can make a chiral molecule is to use something like a chiral auxiliary by auxiliary. We mean that it is a molecule or an attachment to a molecule, which you can remove from the final product and reuse. So, here is an example we have a molecule where we which is pro chiral this is the pro chiral molecule. This is a pro chiral center and you want to transform it to the chiral center which is mentioned here. So, you want to do a substitution and typically you could do this by removing this proton with a base and if you remove that with a base and treat it with an electrophile like R x. So, you have a B minus and then a second reaction with an R x you will end up with the correct molecule. Now, let us say we do it with chiral ether which has got a chiral substituent here, but you only want the phenol. Then you could still do the reaction with this species because you have one chiral center. This chiral center will induce chirality in the position adjacent to it and so you will get very good diastereomeric excesses in this reaction, so one of them is a wanted isomer. You can just hydralize or remove this ether or phenyl aryl ether, hydralize the aryl ether and generate the right product that you want. So, this is something that you can reuse the chiral alcohol that you use to generate the chiral form can be reused. So, in a sense chiral auxiliaries are very useful ways by which we can generate chiral molecules because they can be recycled. Now, chiral reagents are not necessarily a great idea they cannot be used again and again and here is the same example you want to use a base to remove the proton and an R X to do the alkylation. Either R X is coming in can be chiral in which case you can in fact generate the chiral center the diastereomeric molecule is formed because the molecule is now chiral in two places here is a chiral center and here is the other chiral center. So, the molecule can be isolated in an enantiomerically pure form and you could then go ahead with a transformation that you want. In principle either the base or the alkylating agent could be chiral, but notice that the R group that you add on if it has to be stuck with the molecule, you need to worry about the final state of the molecule whether you want an R group there or not in the chiral form. Fortunately, the anion that is generated can recemize easily because it can do an inversion umbrella inversion and become achiral. So, this is not an extremely good example, but nevertheless the 2 examples that I have chosen in the previous slide. In this slide, together they illustrate how chiral regions and chiral auxiliaries could be used. Let us now proceed to the main topic for today’s discussion which is organic synthesis of chiral molecules using organometallic chemistry, that is you can make a organometallic catalyst which is chiral at the metal or at the ligand. So, induce chirality in the reaction that you want to carry out. So, how does a chiral catalyst work, now since chirality will if you have chirality in the molecule in one of the molecules that you are using in the course of the reaction? It can indirectly induce chirality in the reaction that you are carrying out provided, there is a pro chiral center. So, one has to have a pro chiral substrate and either a chiral catalyst or a chiral reagent we have already discussed the chiral reagent. So, let us use a chiral catalyst a combination of these two species will give us an intermediate, which is a diastereomeric intermediate. So, this diastereomeric intermediate or rather we will get two diastereomeric intermediates, and these two diastereomers will be in unequal proportions because unlike enantiomers they will not have the same thermodynamic properties. So, they might be soluble to different extents, they might be formed in different extents and let us label them D 1 and D 2. So, two diastereomeric forms of the molecule the intermediates are formed and one of them will give you the R isomer and the other will give you the S isomer. So, if the two forms are formed in unequal amounts whichever is formed in great amounts will give you the right enantiomer that you are seeking. So, the chiral product can be generated in reasonably good yield in a pure form if you can generate the right diastereomer. So, it is important then to figure out which chiral catalyst would give you the right diastreomer, so that you can get the product that you want. Now, let us proceed with a look at the catalytic site, the catalytic site has for the sake of representation we have again here a schematic representation of catalytic site. It is usually at a metal that the chemistry is happening. So, the site of chemistry or the place where the reaction is happening is the metal. If your ligand is at the position which is far removed from the place, where the reaction is happened it is unlikely that this chirality would be transferred. On the other hand, if you have the region of chirality very close to the reaction center, it is likely that you will transfer more chirality. This is a very simple naive understanding of the whole process, but it tells you the importance of designing the best catalyst and the best metal complex. So, it would be ideal if this metal center itself is chiral. So, here are a few metal centers which can be chiral, let us leave out the D 4 edge, the square planar geometry because that is very difficult to get in a chiral form. Both tetrahedral centers, metal centers and octahedral metal complexes, both of them can be made in a chiral form. So, if you have a metal complex in a tetrahedral geometry or in an octahedral complex, then both of them can be made in a chiral form. They are not many complexes which are inert and which are suitable for carrying out asymmetric catalysis using these molecules using these catalysts. So, typically you have either a chromium carbonyl molecule which has sufficiently labile. It will lose the chirality around the chromium unfortunately, if you have high oxidation state species. They are indeed inert, so you can have inert coordination compounds, but many of them do not support organometallic chemistry because they do not support ligands like alkenes and carbon monoxide and so on. So, we normally do not encounter these systems in organometallic chemistry. Nickel zero for example, is a tetrahedral complex, so one would expect that these molecules could be made chiral at the metal. So, in principle it is possible to generate nickel with 4 different ligands and this should indeed be a chiral molecule. Unfortunately, these molecules are also sufficiently labile and so they will lose chirality after dissociation and a recombination the palladium complexes, which we encounter in catalysis so often are so labile. In fact many of them do not form the 18 electron complex and tend to form the three coordinate, or the two coordinate complexes that I have pictured here. So, making chiral metal complexes turns out to be a daunting task. There are few tetrahedral pseudo tetrahedral complexes which are piano stool complexes. Here the metal has 3 ligands which form the legs of the piano stool and a metallocene, or arene or a cyclo pentadiene which forms a stool part of the molecule and these molecule can indeed be chiral. They can be coordinately saturated if you have the right electron count and for this in the case of iron 2 plus you need 2 anionic ligands. In the case of manganese, a single anionic ligand and you will form these type of molecules which are useful for catalysis. So, there are a few examples where these molecules are chiral at the metal, but the examples are few again because in some instances they can lose the chirality, when they react by losing one of these ligands during the course of catalysis. So, mostly catalysis of organic reactions has to be done with organometallic catalysts which have chiral ligands. So, how does one go about making an organometallic compound with the chiral ligand? Here I have shown for you a slide in which it is indicated that the Nobel Prize for 2001 was in fact given to three people specifically for doing this. They carried out several catalytic asymmetric synthesis, where they used chiral ligands very efficiently and showed that asymmetric catalysis is indeed possible with organometallic chemistry. So, all three of them have carried out studies with various molecules which have got chiral ligands. We will deal with a few of them today and we will look at some of the advancements that have been made in this field as we go along. So, here is a enamide which is in fact a molecule which is extremely important because when the Ar group that is given here. When the Ar group is in fact substituted, the di substituted aromatic ring. If you have C O O H, the acid forms instead of the C O O imine that is mentioned here. If these two changes are made, you are in fact looking at eldopa, the molecule that is used for treating Parkinson’s disease. So, it was very important that you get eldopa and if want to do it you have to do it using a chiral catalyst. It was discovered by one of these people whom I just mentioned that Nobel Prize winner that it will be possible to carry out asymmetric catalysis using this rhodium. Catalyst as a hydrogenation catalyst, the catalytic cycle of this reaction is something that we have already seen. We would not look at it in detail, but sufficed to see that here is a complex where the chirality resides primarily in the phosphine. So, you have a phosphine which is chiral and this ligand is the one which readily leaves the coordination site of the molecule and generated a vacant coordination site, which will lead to subsequent hydrogenation reactions. So, this reaction could be carried out with hundred percent chemical yields. The hydrogenation of this molecule at extremely mild conditions and with very high enantiomeric excess look at the enantiomeric excess that has been achieved is close to 99 percent. This means you have a practically pure drug at the end of this hydrogenation and that too it is in the chiral form. So, how exactly does this reaction work, as I just mentioned to you this cyclo octadiene, 1, 5 cyclo octadiene readily leaves the coordination sphere of the metal and you generate a vacant coordination site. It is a place where the ligand where the yield the alkene moiety is linked to the rhodium, now turns out that there are two phases to this alkene. It can either coordinate the way in which I have shown to you or it can rotate about this axis and coordinate in a slightly different fashion. Let us look at a closer representation of that in a in a minute here I have shown for you the molecule which is coordinated to the rhodium. The same rhodium could be coordinated in a slightly different form it could also be coordinated in such a way. The molecule has got its coordinates in such a way that you have the C O O M e on the right side and the N H C O M e on the left side instead of it presses my right side, and left side these two places could be interchanged. So, you could have coordination of the rhodium in this fashion. So, this type of interaction would give you the diastereomeric form and you would end up with the other isomer. So, the chiral enamide hydrogenation reaction has been used as a test case for many chiral phosphenes. People take a variety of chiral phosphenes and carry out this reaction to see what enantiomeric excesses you achieve, and that is taken as the mark of the efficiency of the phosphine. In this reaction you need a chelating phosphene because during the course of this reaction one arm of the phosphorous can come out, and so if you have two different phosphorous ligands, you tend to lose enantiomeric excess. So, it turns out that this simple ligand that I have shown for you here which is called chirophos is the one which is most efficient and generates the maximum enantiomeric excess. The same reaction that I have just shown for you could be carried out with a variety of other ligands and I have to check for you so many four different ligands here. You notice that many of them are lot more complicated, or more sterically hindered than chirophos, but unfortunately none of them as efficient as chirophos. Chirophos has got this simple metal substituent in the back bone and 2 phenyl groups and still it turns out to be the most efficient system for carrying out asymmetric hydrogenation of alpha acetylaminocinamic acids. This is the base reaction for the generation of eldopa, so it is not clear that you need to have very bulky substituents on this chiral ligands. Nevertheless, there are there appears to be some importance to the presence of phenyl group in the ligand. So, here are the two isomers pictured, now let us look at the theory behind why we get different amounts of the 2 enantiomers. Here are the two diastereomers which are formed in the presence of the rhodioum 1 compound. As soon as you form the complex, you have diastereomer 1 and diastereomer 2. These are the two diastereomers that we are talking about and these diastereomers have two vacant sites. So, depending on the energy difference of these two diastereomers, you will end up with different amounts of R and S enantiomers. So, as I told you before, the energy differences between these two diastereomers, if it is very large you will get only one form of one of the enantionmers because the energy required passing over the hill on the left hand side is more. So, you will uniquely get only the S isomer as a result of this reaction. So, this is a simple enough explanation, but in real life the situation it is little more complicated. After we form the diastereomer 1 you have to add oxidatively add hydrogen. You carry out an oxidative addition of hydrogen and so you get diastereomer 3 and diastereomer 4. These two diastereomers are simply different from the first two diastereomers the fact that there are two hydrogens added on to it. The energy difference between the two diastereomers, diastereomer 3 and diastereomer 4 need not be the same or need not be even in the same direction as the energy differences between diastereomer 1 and 2. So, in the real life situation, there are two different diastereomers and I have given you an example which is a favorable situation. Suppose, you have this reaction coordinate where this is your starting material and you go through the diastereomer 1 and the diastereomer 3, and you form the isomer R clearly going towards the right side of this reaction. Coordinate is favorable and going towards the left side to form the s isomer turns out to be less favorable you will get a very good yield of R. Sometimes, the situation is really different and you get a complication where diastereomer 1 is more difficult to form and diasteremer 3 is easy whereas diastereomer 4 is higher in energy that diastereomer 3. So, as a result you have a complicated situation and the percentage if R and S, you achieve will be different from the situation that I described to you a little earlier. So, fortunately for us there is something called as a curtain hammer principle which suggests that if you have a small energy barrier for the formation of D 1 and D 2. The net difference, only the net difference between D 3 and D 4 are important. So, it is in fact only this energy becomes important, if these two energy barriers are fairly small. Then curtain hammer principle suggests that the energy difference between D 3 and D 4 will help us decide between ratios of S and R that are formed. Unfortunately, if D 1 energy required for passing over the two barriers are comparable then you tend to have a very complicated situation. So, the moral of the story is as the number of intermediates involved increase, then the predictability goes down and not only is the predictability difficult, the probability of getting both isomers increase. In other words, you might get a mixture of R and S and these are the real life situations, which we encounter and as a result you end up with very difficult situations in trying by trying to make one chiral form of the molecule. Let us take just one other example before we discuss some of the principles which are behind these chiral ligands are behind designing these chiral ligands. Here is an example where you do hydrosilylation and subsequent hydrolysis. So, this results in the hydrolysis in the reduction of this ketone. The ketone is marked here, so it is a methyl ketone that we are talking about. We end up with very good enantiomeric excess reasonably good 76 percent enantiomeric excess and extremely good yield, when you reduce it with the silane, so this hydrosilylation is again catalyzed by the same rhodium complex. So, we can carry out this reaction by using a chiral ligand and this is the chiral ligand that we need to use is again a diphosphene. It is convenient to use a diphosphene and generate the chiral form of the alcohol. Once again, this reaction requires that we have a chelating ligand, and several ligands have been used in addition to what I have just shown you in the previous transparent sheet. In the previous projections where you had two phosphenes I have for you 2 different ligands. Here, one is called DIOP and it is a very popular ligand, it is derived from tartaric acid, and so it is very accessible in the chiral form tartaric acid is readily available in the chiral form in nature. So, you can make this ligand in fairly large quantities and it is a very useful ligand to have here is another ligand which appears to be much simpler. This ligand has also been used and two equivalents of this ligand because there are only one phosphorous two equivalents can be used in order to induce chirality. So, let us take a look at the table of a ligand and the type of enantiomeric excesses that you obtain when you have a change in the substrate. When you have a change in the ligand, the reaction is the same. It is the reduction of a ketone to an alcohol and this conversion can be carried out with a variety of different silanes, and depending on the silane you get different enantiomeric excesses. Depending on the ligand that we use, the phosphorous ligand that you use you tend to have different amounts of enantiomeric excess. Now, if you closely look at this table, the first example is in fact using the same chiral ligand. So, here is an example where the same chiral ligand has been used, only difference between the two is that you used a different reducing agent, the substrate is the same different reducing agent is used and change the configuration of the product. So, in other words depending on the reagent that you used that you used for the reduction reaction, you can control the product although you have not changed the chirality at the asymmetric catalyst center. You change the chirality of the product that is being formed, so this is something which could not have predicted and it seems to be something that is dependent on inherent to the inherent to the system. So, one has to study each the reagent and each substrate and then decide which is the reagent that you want to use. So, here are another example 2 molecules, and again the same ligand, so these 2 ligands are also the same and using the same reducing agent, the only difference between these 2 substrate is the fact that you have a C O O R group that is present on the C O. So, you end up with a group which has got the ability to coordinate, so C O O M e group is able to coordinate through the incoming region which is the silicon hydride. So, the secondary coordination turns out to be extremely good in increasing the enantio selectivity of the part. So, you can expect increased enantio selectivity when you have secondary interactions between the ligand and your substrate between the substrate and the reagent. So, these are factors which we have to look on for, so let us look at one other comparison which is the last. Here, you use the same chiral ligand with a different sense, so here is a so here is A plus BMPP ligand and A minus BMPP ligand. The same reagent is used and the same substrate is used and you expect the opposite chirality for the product and that is also achieved. So, here you get as product in the second case you get the r product, but notice the enantiomeric excess is not identical, so this tells you that the diastreomeric ratios that you have are because they are not identical enantiomeric excesses in a reaction. So, asymmetric catalysis turns out to be an extremely challenging task, one has to study each reaction carefully. And we will discuss this with some terminologies and techniques that have come about. Let us take a look at the chiral ligand, this time we have in fact shown you two reactions, one is the hydrogenation and another is the hydrosilylation and both instances we used phosphorous ligands. These phosphorous ligands have been made chiral not because of the chirality at phosphorous in always, but because of the chirality in the backbone of the chain which is attached to the phosphorous atoms. So, what are some principle key features that we will use when you want to design a chiral ligand? Here is a list, we will not look at examples for each one of them, but we will take a look at some of these principles which are used in order to generate good chirality induction. The first one is of course whether it should be a chelating ligand or a non-chelating ligand. This really depends on the reaction and the catalyst, the catalyst loses a phosphorous ligand during the course of the reaction or during one part of the catalytic cycle which is involved in asymmetric induction. Then it is important that we use a chelating ligand, this we illustrated in the first reaction for the hydrogenation reaction. It is very important that you have a chelating ligand, but this because it was one of the first reactions which were studied extensively. People have tended to believe that we should always have a chelating ligand, and that is not necessarily true the ligand byte size was different in the case of chirophos and in DIOP and that made a difference in the amount of chirality. It induces a second factor, that if there are two ligands and the two ligands and both ligands are connected to one another. Then if they are going to have A C 2 symmetry the number of intermediates would come down by half. So, having C 2 symmetry in the ligand structure turns out to be a way in which you can reduce the number of intermediates and so increase chirality only requires the absence of an S n or asymmetry. So, one can have C 2 symmetry and still one can have very good chirality induction. Now, comes secondary coordination which I have already hinted for you. In the previous transparent sheet if you have weak interactions between the ligand and the reagent. If you have between the substrate and the reagent like hydrogen bonding or electrostatic interaction or pi interaction as in the case of two phenyl groups on the substrate and the reagent. Then it turns out that enantiomeric excesses will be higher enantiomeric excesses go up, when you have any interaction between these 3 groups of my which are interacting steric crowding. Although it is not a key factor, it is important when you want to increase the chirality at least in some cases steric crowding is as important as the secondary coordination. Lastly, we should emphasize the fact that in order to generate these chiral molecules, we need a pool of chiral molecules are these are provided by naturally occurring sugars and amino acids and peptides. So, these molecules provide chiral pool this has been called the chiral pool approach for asymmetrical catalysis and asymmetric ligand design. As I mentioned to you it is important to have a chiral ligand but not always here is an example which has been very efficiently illustrated by Rajan Babu who has studied these sugar coded phosphenes. Here is allopyranoside, these are two sugar moieties which are linked to each other and one of the alcohol groups has been converted into a phosphine. So, you can either have a phosphite or a phosphinite depending on the type of groups that you have here, I will mark it with a different color in order to emphasize that. So, here is the group which is chiral this is the chiral moiety and this chiral moiety can be either be a phosphate, or a phosphinite and depending on the reaction one might have to use either a phosphite of a phosphinite the electronic requirements are different ,and this has been amply illustrated by Rajan Babu for conditions. So, there are other reactions which have been studied by Reetz who also used monodentate phosphites, and has shown these are significant enough for sufficient for carrying out chirality induction very efficiently. A major challenge comes up with asymmetric acyclic activation, these are especially difficult because asymmetric activation requires that we have some region selectivity. So, R and R dash are different, whether you want to carry out a substitution in this position is determined by the ligand that is there. Then palladium turns out to be an extremely useful metal, and palladium phosphorous complex is turn out to be very good for carrying out the activation of these acyclic substrates towards nucleophilic substitution reactions. One of the nucleophiles is commonly used is the diethyl malenate substrate and so here is the diethyl malenate group, which is which comes in and whether it acts in the position in the first position or in the second position. It is critically determined by the ligand which is there on the palladium, and secondly depending on how you have a chiral moiety on the phosphorous. It determined whether the molecule the final product is chiral, or not in this context is has been important to generate what are called modular ligand systems, this is a same idea which has been called differently by different people. Some people have called it the molecular tool box technique, or editing the steric chemical elements all of them are nice catch words. They are based on the simple idea that if you have a phosphinite or a phosphite, you can vary the influence of each group independently by changing the R groups. For example, in this case in this in this phosphorous ligand here there are three different handles, which very easily changes in the binol which is chiral, which is coordinated to the phosphorous. You could either change R or R dash and you could also change in the moiety that is attached to the phosphorous. You can change this R group, so you have three different combinations that can be varied. And you will very soon have a large pool of chiral molecules and the large number of chiral molecules can be used as a test for testing the acyclic activation reaction. Here is another possibility, I have already shown you sugars and how Rajan Babu has used sugars for coating phosphorous, or covering the phosphorous ligands with them and because they are chiral they induce chirality in the product. Hoveyda has specialized in the use of peptide ligands, so here is a ligand which is based on a peptide. There are two amino acids linked together and here are the two amino acids which are linked together C O N H group C O N H group. These two amino acids are now linked to an aromatic phosphene and this now turns out to be a catalyst because it can coordinate to cooper 1. The reagent that is used for alkylation of these enone the R group attacks in this position, and the final product turns out to be chiral becuase this is a pro chiral center where you are going to add the R group. This 1, 4 additions are usually carried out by a copper organometallic and this copper organometallic will have a phosphorous ligand attached to it, when it carries out this nucleophilic substitution. You can have a large number of rings that can be alkylated in these places and Hoveyda is exploited that to make a chiral naturally molecule, which is naturally occurring molecule is biologically active. He has made it in 97 percent enantiomeric excess by using this ligand which is again based on a peptide which is available, so it turns out that you can either sugars or peptides and convert them into phosphenes or phosphites. These are in turn very convenient for carrying out this type of asymmetric catalysis. Now, you might be wondering how you decide which ligand we can use for which reaction. Now, there are companies who encourage you to take a look at this company’s website, which in fact has a list of reactions which are which can be carried out using chiral ligands which are available in the literature. They suggest what ligand can be used for the particular reaction. So, here are the list of reactions the hydrogenation reaction that need to be carried out, and the enantiomeric excesses that can be expected from these reaction if you use the preferred catalyst. So, these catalysts have been developed by various researchers like Rajan Babu and the others that I have mentioned. It is now possible to go to the literature and figure out which ligand, I should check first before I start a research project on say the reduction of C N groups or for alkylation of an enone and so on. So, before we close we should mention that until now we have dealt with main group. We have dealt with we have been talking about transition metal organometallic catalysis. Now, is it possible to have main group metals catalyzing reactions in asymmetric fashion? So, asymmetric catalysis or A C can be in fact carried out with main group or organometallic compounds. Here, I have for you an example where the calcium compound which is listed here this one here is to start with it is the solvent molecule which is either T H F or dimethyl ether. It is replaced by the chiral ligand, which I have for you on the right side of the screen. This DIOL which can be made in a in an easy fashion in a chiral form can be used as a ligand for this calcium compound before it catalyzes. This aldol reaction very efficiently in a chiral form, so here is the chiral center which has been generated in the aldol. The chirality is extremely high when you use these calcium complex complexes with the chiral ligand. Now, it turns out that in solution has been possible for them to show that there is complex calcium a calcium coordination compound in which you have the ligand associated with the calcium. Unfortunately, the ratio is not 1 is to 1 5. Calcium atoms are associated merely with 8 of these bases or the alcohols in the structure of the species which has been detected in the mass spectrometer. This is only an indication that it is possible to have calcium coordinated with these DIOL’s in solution. The inference that you have a chiral product, suggests that somehow this chiral ligand must have played a rule during the aldol condensation of these two reactants. So, although we have discussed a variety of principles here, we notice that there is a long way to go. People have developed various ligands structures each ligand structures have got complicated geometries, but there is no simple way to predict that if you take one particular ligand you will be able to induce a maximum chirality. Merely increasing the bulk of the ligand does not help in inducing chirality by adding hydrogen bonding groups. For that matter pi, pi stacking interactions does not always give you the best chirality. One more importantly, it is not a predictable change although there are increases in the chirality induction, when you add hydrogen bonding groups or pi, pi stacking interactions. So, there is a lot to study and lot to contribute in this particular area.
Preparation
Phosphinites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of chlorodiphenylphosphine with methanol and base gives methyl diphenylphosphinite:
- ClPPh2 + CH3OH → CH3OPPh2 + HCl
Although they are esters of phosphinous acids (R2POH), phosphinites are not made via such intermediates.
Reactions
Oxidation of phosphinites gives phosphinates:
- 2 P(OR)R2 + O2 → 2 OP(OR)R2
Phosphinites are ligands, giving derivatives similar to metal phosphine complexes. They are stronger pi-acceptors than typical phosphine ligands.[2]
References
- ^ D. E. C. Corbridge (1995). Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- ^ Rajanbabu, T. V. Babu (2012). "Phosphinite and Phosphonite Ligands". Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis. pp. 159–232. doi:10.1002/9781118299715.ch5. ISBN 9781118299715.
See also
- Phosphine - PR3
- Phosphine oxide - OPR3
- Phosphonite - P(OR)2R
- Phosphite - P(OR)3
- Phosphinate - OP(OR)R2
- Phosphonate - OP(OR)2R
- Phosphate - OP(OR)3
