 | |
| Clinical data | |
|---|---|
| Other names | PEPAP |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
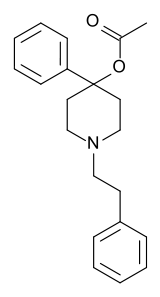
| Formula | C21H25NO2 |
| Molar mass | 323.436 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
PEPAP (phenethylphenylacetoxypiperidine) is an opioid analgesic that is an analog of desmethylprodine.
It is related to the drug MPPP, with an N-phenethyl group in place of the N-methyl substitution and an acetate ester rather than propionate. PEPAP is approximately 6–7 times more potent than morphine in laboratory rats.[1] PEPAP presumably has similar effects to other opioids, producing analgesia, sedation and euphoria. Side effects can include itching, nausea and potentially serious respiratory depression which can be life-threatening.
PEPAP has been found to be a potent CYP2D6 inhibitor, which makes it likely to cause adverse interactions with some other drugs, although the inhibitory potency of PEPAP is less than that of MPPP.[2] Both cocaine and methadone are also CYP2D6 inhibitors and could, in theory, potentiate the effect.
It is unlikely that the tetrahydropyridine byproducts that may be formed during the synthesis of PEPAP are neurotoxic in the same way as the MPPP byproduct MPTP. It appears that the N-methyl group of MPTP is required for neurotoxic activity. In animal experiments, only MPTP analogues that preserved the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine structure were active as dopaminergic neurotoxins. Most structural changes, including replacing the N-methyl group with other substituents, abolished neurotoxicity.[3]
There is evidence that the clandestine manufacturers who produced MPPP in the 1970s, including the tainted batch, went on to produce PEPAP[4] in an attempt to avoid using watched precursors or drug intermediates that were illegal.
YouTube Encyclopedic
-
1/5Views:223 39139 604 14316 275 383399 0854 831 555
-
Peppa Pig Full Episodes 🔴 LIVE! BACK TO SCHOOL 📚 Cartoons for Kids
-
Peppa Pig English Full Episodes Compilation #120
-
Peppa Pig Gets A New Environmentally Friendly Electric Car 🐷🚗 Peppa Pig Official Family Kids Cartoon
-
Peppa Pig Full Episodes 🔴 LIVE! Peppa Pig SPECIAL EPISODES - Cartoons for Kids
-
English Cartoon | Peppa Pig English Episodes - Compilation 2 - Peppa Pig Episodes
Transcription
See also
References
- ^ Janssen PA, Eddy NB (February 1960). "Compounds related to pethidine-IV. New general chemical methods of increasing the analgesic activity of pethidine". Journal of Medicinal and Pharmaceutical Chemistry. 2: 31–45. doi:10.1021/jm50008a003. PMID 14406754.
- ^ Pritzker D, Kanungo A, Kilicarslan T, Tyndale RF, Sellers EM (June 2002). "Designer drugs that are potent inhibitors of CYP2D6". Journal of Clinical Psychopharmacology. 22 (3): 330–332. doi:10.1097/00004714-200206000-00015. PMID 12006905. S2CID 492513.
- ^ Youngster SK, Sonsalla PK, Sieber BA, Heikkila RE (June 1989). "Structure-activity study of the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity. I. Evaluation of the biological activity of MPTP analogs". The Journal of Pharmacology and Experimental Therapeutics. 249 (3): 820–828. PMID 2786564.
- ^ Langston JW, Palfreman J (1995). The Case of the Frozen Addicts. Pantheon Books. ISBN 0-679-42465-2.
