| |
| Names | |
|---|---|
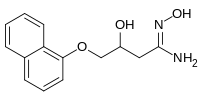
| IUPAC name
N′,3-Dihydroxy-4-naphthalen-1-yloxybutanimidamide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | C006154 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H16N2O3 | |
| Molar mass | 260.293 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nadoxolol is an antiarrhythmic agent (i.e., a drug for the treatment of irregular heartbeat),[1] chemically related in structure to beta-adrenergic receptor blocker drugs such as propranolol.[2]
It does not appear to be marketed anywhere in the world.
YouTube Encyclopedic
-
1/1Views:5 263
-
Naphthalene Organic Chemistry: Synthesis, Chemical Reactions and Uses | Polynuclear Hydrocarbons
Transcription
References
- ^ Warembourg, H; Ducloux, G (1976). "Clinical study of a new anti-arrhythmia agent: nadoxolol". Lille Med. 21 (4): 386–8. PMID 957857.
- ^ Kapoor, M; Anand, N; Koul, S; Chimni, SS; Manhas, KS; Raina, C; Parshad, R; Taneja, SC; Qazi, GN (2003). "Kinetic resolution of 1-chloro-3-(1-naphthyloxy)-2-propanol, an intermediate in the synthesis of beta-adrenergic receptor blockers". Bioorganic Chemistry. 31 (3): 259–69. doi:10.1016/s0045-2068(03)00050-6. PMID 12818235.
