| |
| Names | |
|---|---|
| IUPAC name
Lithium oxalate
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.232 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li2C2O4 | |
| Molar mass | 101.90 g·mol−1 |
| Appearance | Colorless crystalline solid |
| Density | 2.12 g/cm3 |
| 6.6 g per 100 g of water | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312 | |
| P264, P270, P280, P301+P312, P302+P352, P312, P322, P330, P363, P501 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
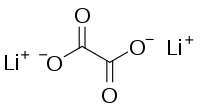
Lithium oxalate is an organic compound with the chemical formula Li2C2O4. It is a salt of lithium metal and oxalic acid.[3][4] It consists of lithium cations Li+ and oxalate anions C2O2−4. Lithium oxalate is soluble in water and converts to lithium oxide when heated.[5]
YouTube Encyclopedic
-
1/5Views:16 3382 88976 73950 46016 492
-
How to Write the Formula for Lithium oxide
-
How to Write the Formula for Lithium carbonate
-
Lithium from Phone (Li-ion) Batteries - Part 2: Li2CO3
-
How to Balance Li + O2 = Li2O (Lithium + Oxygen gas)
-
ANOMALOUS BEHAVIOUR OF LITHIUM PART_ 01
Transcription
Synthesis
One of the methods of synthesis is the reaction of direct neutralization of oxalic acid with lithium hydroxide:
- 2 LiOH + H2C2O4 → Li2C2O4 + 2 H2O
Properties
The compound crystallizes in the monoclinic system, cell parameters a = 3.400 Å, b = 5.156 Å, c = 9.055 Å, β = 95.60°, Z = 4.[3]
Lithium oxalate decomposes when heated at 410–500 °C (770–932 °F; 683–773 K):
- Li2C2O4 → Li2CO3 + CO
Applications
In pyrotechnics, the compound is used to color the flame red.[6]
References
- ^ "553-91-3 | Sigma-Aldrich". Sigma Aldrich. Retrieved 15 June 2021.
- ^ "di-Lithium oxalate". Merck Millipore. Retrieved 15 June 2021.
- ^ a b Beagley, B.; Small, R. W. H. (1964-06-10). "The structure of lithium oxalate". Acta Crystallographica. 17 (6): 783–788. doi:10.1107/S0365110X64002079. Retrieved 15 June 2021.
- ^ Solchenbach, Sophie; Wetjen, Morten; Pritzl, Daniel; Schwenke, K. Uta; Gasteiger, Hubert A. (2018). "Lithium Oxalate as Capacity and Cycle-Life Enhancer in LNMO/Graphite and LNMO/SiG Full Cells". Journal of the Electrochemical Society. 165 (3): A512–A524. doi:10.1149/2.0611803jes. S2CID 104199908.
- ^ "Lithium Oxalate". Millipore-Sigma. Retrieved 10 Feb 2022.
- ^ Koch, Ernst-Christian (2009). Is it possible to Obtain a Deep Red Pyrotechnic Flame Based on Lithium?. 36th International Pyrotechnics Seminar. doi:10.13140/2.1.1657.0567. Retrieved 15 June 2021.
