 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eloxatin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607035 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Complete |
| Elimination half-life | ~10 – 25 minutes[4] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.150.118 |
| Chemical and physical data | |
| Formula | C8H14N2O4Pt |
| Molar mass | 397.294 g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
Oxaliplatin, sold under the brand name Eloxatin among others, is a cancer medication (platinum-based antineoplastic class) used to treat colorectal cancer.[5] It is given by injection into a vein.[5]
Common side effects include numbness, feeling tired, nausea, diarrhea, and low blood cell counts.[5][6] Other serious side effects include allergic reactions.[6][5] Use in pregnancy is known to harm the baby.[5] Oxaliplatin is in the platinum-based antineoplastic family of medications.[7] It is believed to work by blocking the duplication of DNA.[5]
Oxaliplatin was patented in 1976 in Japan and approved for medical use in 1996 in Europe.[8] It is on the 2023 World Health Organization's List of Essential Medicines.[9]
YouTube Encyclopedic
-
1/5Views:7475803 17455020 858
-
Dr. Axel Grothey Examines the Use of Adjuvant Oxaliplatin in Colon Cancer
-
A promising future for gemcitabine-oxaliplatin in cisplatin-unfit advanced urothelial carcinoma
-
Reducing the neurotoxic side effects of adjuvant chemotherapy for colon cancer
-
MSI in patients with stage III colon cancer receiving 5-FU with or without oxaliplatin
-
Side Effects of Chemotherapy Options in Colorectal Cancer
Transcription
Medical uses
Oxaliplatin is used for treatment of colorectal cancer, typically along with folinic acid (leucovorin) and fluorouracil in a combination known as FOLFOX[10] or along with capecitabine in a combination known as CAPOX[11] or XELOX.[12] It may also be effective against breast cancer, germ cell tumor, ovarian cancer, non-small-cell lung cancer, and pancreatic cancer.[13]
Advanced colorectal cancer
Oxaliplatin by itself has modest activity against advanced colorectal cancer.[14] When compared with just 5-fluorouracil and folinic acid administered according to the de Gramont regimen, a FOLFOX4 regime produced no significant increase in overall survival, but did produce an improvement in progression-free survival, the primary end-point of the phase III randomized trial.[15]
Adverse effects
Side-effects of oxaliplatin treatment can potentially include:
- Neurotoxicity leading to chemotherapy-induced peripheral neuropathy, a progressive, enduring and often irreversible tingling numbness, intense pain and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs, often with deficits in proprioception.[16] This chronic neuropathy may also be preceded by a transient acute neuropathy occurring at the time of infusion and associated with excitation of voltage-gated Na+ channels.[17][18]
- Fatigue
- Nausea, vomiting, or diarrhea
- Neutropenia (low number of a type of white blood cells)
- Ototoxicity (hearing loss)
- Extravasation if oxaliplatin leaks from the infusion vein it may cause severe damage to the connective tissues.
- Hypokalemia (low blood potassium), which is more common in women than men[19]
- Persistent hiccups[20]
- Rhabdomyolysis[21]
In addition, some patients may experience an allergic reaction to platinum-containing drugs. This is more common in women.[19]
Oxaliplatin has less ototoxicity and nephrotoxicity than cisplatin and carboplatin.[16]
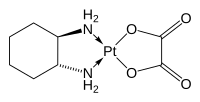
Structure and mechanism
The compound features a square planar platinum(II) center. In contrast to other drugs of the platinum-based antineoplastic class of drugs cisplatin and carboplatin, oxaliplatin features the bidentate ligand trans-1,2-diaminocyclohexane in place of the two monodentate ammine ligands. It also features a bidentate oxalate group.[7] The three-dimensional structure of the molecule has been elucidated by X-ray crystallography, although the presence of pseudosymmetry in the crystal structure has caused confusion in its interpretation.[22]
According to in vivo studies, oxaliplatin fights carcinoma of the colon through non-targeted cytotoxic effects. Like other platinum compounds, its cytotoxicity is thought to result from inhibition of DNA synthesis in cells. In particular, oxaliplatin forms both inter- and intra-strand cross links in DNA,[23] which prevent DNA replication and transcription, causing cell death.
History
Oxaliplatin was first synthesized in 1978 at Nagoya City University by Yoshinori Kidani.[24] It was later developed in Europe as a less toxic and more effective alternative to cisplatin. It gained European approval in 1996,[25] and approval by the U.S. Food and Drug Administration in 2002.[26] Generic oxaliplatin was first approved in the United States in August 2009.[27] Patent disputes caused generic production to stop in 2010, but it restarted in 2012.[28][29]
Patent information
Eloxatin was covered by patent numbers 5338874 (expired 7 April 2013), 5420319 (expired 8 August 2016), 5716988 (expired 7 August 2015) and 5290961 (expired 12 January 2013) (see Electronic Orange Book patent info for Eloxatin).[30] Exclusivity code I-441, which expired on 4 November 2007, is for use combination with infusional 5-FU/LV for adjuvant treatment stage III colon cancer patients who have undergone complete resection primary tumor-based on improvement in disease free survival with no demonstrated benefit overall survival after 4 years. Exclusivity code NCE, New Chemical Entity, expired on 9 August 2007.[30]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ "Eloxatin- oxaliplatin injection, solution, concentrate". DailyMed. 22 October 2019. Retrieved 26 May 2022.
- ^ Ehrsson H, Wallin I, Yachnin J (2002). "Pharmacokinetics of oxaliplatin in humans". Medical Oncology. 19 (4): 261–265. doi:10.1385/MO:19:4:261. PMID 12512920. S2CID 1068099.
- ^ a b c d e f "Oxaliplatin". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ a b Oun R, Moussa YE, Wheate NJ (May 2018). "The side effects of platinum-based chemotherapy drugs: a review for chemists". Dalton Transactions. 47 (19): 6645–6653. doi:10.1039/c8dt00838h. PMID 29632935.
- ^ a b Apps MG, Choi EH, Wheate NJ (August 2015). "The state-of-play and future of platinum drugs". Endocrine-Related Cancer. 22 (4): R219–R233. doi:10.1530/ERC-15-0237. hdl:2123/24426. PMID 26113607.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 513. ISBN 9783527607495. Archived from the original on 20 December 2016.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "FOLFOX". National Cancer Institute. 18 September 2009. Retrieved 26 May 2022.
- ^ "CAPOX". National Cancer Institute. 4 April 2012. Retrieved 26 May 2022.
- ^ "XELOX". National Cancer Institute. 6 January 2012. Retrieved 26 May 2022.
- ^ Townsend D (2007). "Oxaliplatin". xPharm: The Comprehensive Pharmacology Reference. Elsevier. pp. 1–4. doi:10.1016/B978-008055232-3.62973-3. ISBN 9780080552323.
- ^ Bécouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, et al. (August 1998). "Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers". Journal of Clinical Oncology. 16 (8): 2739–2744. doi:10.1200/JCO.1998.16.8.2739. PMID 9704726.
- ^ de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. (August 2000). "Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer". Journal of Clinical Oncology. 18 (16): 2938–2947. doi:10.1200/JCO.2000.18.16.2938. PMID 10944126.
- ^ a b Pasetto LM, D'Andrea MR, Rossi E, Monfardini S (August 2006). "Oxaliplatin-related neurotoxicity: how and why?". Critical Reviews in Oncology/Hematology. 59 (2): 159–168. doi:10.1016/j.critrevonc.2006.01.001. PMID 16806962.
- ^ Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A (December 2005). "Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels". British Journal of Pharmacology. 146 (7): 1027–1039. doi:10.1038/sj.bjp.0706407. PMC 1751225. PMID 16231011.
- ^ Gebremedhn EG, Shortland PJ, Mahns DA (April 2018). "The incidence of acute oxaliplatin-induced neuropathy and its impact on treatment in the first cycle: a systematic review". BMC Cancer. 18 (1): 410. doi:10.1186/s12885-018-4185-0. PMC 5897924. PMID 29649985.
- ^ a b Chay WY, Chew L, Yeoh TT, Tan MH (May 2010). "An association between transient hypokalemia and severe acute oxaliplatin-related toxicity predominantly in women". Acta Oncologica. 49 (4): 515–517. doi:10.3109/02841860903464015. PMID 20092386. S2CID 33026126.
- ^ "Oxaliplatin Side Effects". Drugs.com. Archived from the original on 5 September 2014. Retrieved 5 September 2014.
- ^ "Eloxatin information". mein.sanofi.de (in German). Archived from the original on 27 August 2016. Retrieved 15 June 2016.
- ^ Johnstone TC (January 2014). "The Crystal Structure of Oxaliplatin: A Case of Overlooked Pseudo Symmetry". Polyhedron. 67: 429–435. doi:10.1016/j.poly.2013.10.003. PMC 3885251. PMID 24415827.
- ^ Graham J, Mushin M, Kirkpatrick P (January 2004). "Oxaliplatin". Nature Reviews. Drug Discovery. 3 (1): 11–12. doi:10.1038/nrd1287. PMID 14756144.
- ^ Pizarro AM, Barry NP, Sadler PJ (2013). "3.25 - Metal–DNA Coordination Complexes". Comprehensive Inorganic Chemistry II (2nd ed.). Elsevier. pp. 751–784. doi:10.1016/B978-0-08-097774-4.00330-2. ISBN 9780080965291.
- ^ Janjan NA, Delclos ME, Crane CH, Krishnan S, Das P (2010). "Chapter 24 - The Colon and Rectum". Radiation Oncology (9th ed.). Mosby. pp. 560–605. ISBN 978-0-323-04971-9.
- ^ "Eloxatin FDA Approval History". Drugs.com.
- ^ "Generic Eloxatin availability". Drugs.com. Archived from the original on 7 June 2013. Retrieved 19 April 2014.
- ^ "Hospira Announces U.S. Re-Launch Of Generic Oxaliplatin Injection" (Press release). Archived from the original on 24 September 2015. Retrieved 25 August 2015.
- ^ "Top 10 best-selling cancer drugs: Eloxatin–$1.2 billion". FiercePharma. 15 May 2012. Archived from the original on 21 April 2014. Retrieved 20 April 2014.
- ^ a b "Patent and Exclusivity Search Results from query on Appl No 021759 Product 001 in the OB_Rx list". Orange Book. U.S. Food and Drug Administrartion. Archived from the original on 26 September 2007.. Accessed on: 22 July 2007.
Further reading
- Graham J, Mushin M, Kirkpatrick P (January 2004). "Oxaliplatin" (PDF). Nature Reviews. Drug Discovery. 3 (1): 11–12. doi:10.1038/nrd1287. PMID 14756144. Archived from the original (PDF) on 8 November 2004. Retrieved 19 July 2005.
External links
- "Oxaliplatin". National Cancer Institute. 5 October 2006.
