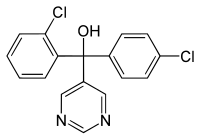
Names
IUPAC name
(R /S )-2,4′-Dichloro-α-(pyrimidin-5-yl)benzhydryl alcohol
Other names
α-(2-Chlorophenyl)-α-(4-chlorophenyl)-5-pyrimidinemethanol
Identifiers
ChEBI
ChemSpider
ECHA InfoCard 100.056.432
UNII
InChI=1S/C17H12Cl2N2O/c18-14-7-5-12(6-8-14)17(22,13-9-20-11-21-10-13)15-3-1-2-4-16(15)19/h1-11,22H
Key: NHOWDZOIZKMVAI-UHFFFAOYSA-N
InChI=1/C17H12Cl2N2O/c18-14-7-5-12(6-8-14)17(22,13-9-20-11-21-10-13)15-3-1-2-4-16(15)19/h1-11,22H
Key: NHOWDZOIZKMVAI-UHFFFAOYAN
Clc1ccc(cc1)C(O)(c2ccccc2Cl)c3cncnc3
Properties
C 17 H 12 Cl 2 N 2 O
Molar mass
−1
Appearance
Colorless powder with aromatic odor
Melting point
117 to 119 °C (243 to 246 °F; 390 to 392 K)[1]
Boiling point
240 °C (464 °F; 513 K) (decomposition)[1]
13.7 mg/L at 25 °C
Solubility in other solvents
Soluble in acetone, xylene and methanol[1]
Vapor pressure
65 μ Pa (25 °C)[1]
Hazards
Lethal dose or concentration (LD, LC):
>2000 mg·kg−1 (oral, Rat)[1]
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Fenarimol , sold under the tradenames Bloc , Rimidin and Rubigan , is a fungicide which acts against rusts , blackspot and mildew fungi. It is used on ornamental plants , trees, lawns , tomatoes, peppers, eggplants, cucumbers and melons . It is mainly used to control powdery mildew. It works by inhibiting the fungus's biosynthesis of important steroid molecules (via blockade of the CYP51 enzyme).[2]
YouTube Encyclopedic
Problema 7. Proponer la mejor ruta sintética para cada uno de los alcoholes.
History Fenarimol was developed by Eli Lilly & Company around 1971.[3]
As of early 2018, derivatives of this compound are being researched in an open source manner for possible treatment of eumycetoma .[4]
Synthesis Fenarimol is made by the reaction of 2,4'-dichlorobenzophenone with an organolithium pyrimidine made via bromine-lithium exchange.[2]
References
^ a b c d e EU-Data .^ a b Clayden J, Greeves N, Warren S (2005). Organic chemistry 216 . ISBN 978-0-19-850346-0 ^ GB 1218623 ^ Reynolds, Todd B.; Lim, Wilson; Melse, Youri; Konings, Mickey; Phat Duong, Hung; Eadie, Kimberly; Laleu, Benoît; Perry, Benjamin; Todd, Matthew H.; Ioset, Jean-Robert; van de Sande, Wendy W. J. (2018). "Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma" . PLOS Neglected Tropical Diseases . 12 (4): e0006437. doi :10.1371/journal.pntd.0006437 ISSN 1935-2735 . PMC 5940239 PMID 29698504 .
External links Fenarimol
ER
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel
3β,5α-Dihydrolevonorgestrel
3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin, pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran) MixedSERMs Antagonists
Coregulator-binding modulators: ERX-11
GPER
Agonists Antagonists Unknown
This page was last edited on 12 December 2021, at 12:04