 | |
| Clinical data | |
|---|---|
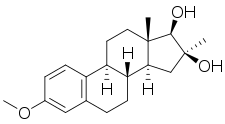
| Other names | SC-6924; Manvene; Anvene; 3-Methoxy-16α-methylestra-1,3,5(10)-triene-16β,17β-diol; 16α-Methylestriol 3-methyl ether; 16β-Hydroxy-16α-methylestradiol 3-methyl ether |
| Routes of administration | By mouth |
| Drug class | Estrogen; Estrogen ether |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H28O3 |
| Molar mass | 316.441 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mytatrienediol (developmental code name SC-6924; former tentative brand names Manvene, Anvene), also known as 16α-methyl-16β-epiestriol 3-methyl ether or 16β-hydroxy-16α-methylestradiol 3-methyl ether, is a synthetic steroidal estrogen medication and an estrogen ether which was derived from estriol and was developed for clinical use in the late 1950s but was never marketed.[1] It was investigated as a weak and mildly estrogenic medication for men to treat atherosclerosis, improve serum lipid profiles, and reduce the risk of myocardial infarction.[2][3][4][5][6][7] However, while preclinical research supported the profile of mytatriendiol as a weak estrogen, the medication was found in clinical trials to produce estrogenic side effects including feminization, breast pain, and gynecomastia in men similarly and comparably to other estrogens such as ethinylestradiol and conjugated estrogens, and its side effects ultimately precluded its use.[8][3][2] The medication was also studied to treat bone pain in patients with multiple myeloma, metastatic bone disease, and osteoporosis, with effectiveness seen.[9]
See also
References
- ^ Pincus G (22 October 2013). Hormones and Atherosclerosis: Proceedings of the Conference Held in Brighton, Utah, March 11-14, 1958. Elsevier Science. pp. 249, 254, 263, 371–374, 411–412, 443–447, 460–461. ISBN 978-1-4832-7064-7.
- ^ a b Marmorston J, Moore FJ, Kuzma OT, Magidson O, Weiner JM (1963). "Effect of Estrogens on Interlipid Relations in Men with Myocardial Infarction". Experimental Biology and Medicine. 113 (2): 357–361. doi:10.3181/00379727-113-28365. ISSN 1535-3702. S2CID 72677634.
- ^ a b Marmorston J, Moore FJ, Hopkins CE, Kuzma OT, Weiner J (June 1962). "Clinical studies of long-term estrogen therapy in men with mvocardial infarction". Proceedings of the Society for Experimental Biology and Medicine. 110 (2): 400–408. doi:10.3181/00379727-110-27531. PMID 14470097. S2CID 24669631.
- ^ Davis FW, Scarborough WR, Mason RE, Singewald ML, Baker BM (January 1958). "Experimental hormonal therapy of atherosclerosis: preliminary observations on the effects of two new compounds". The American Journal of the Medical Sciences. 235 (1): 50–59. doi:10.1097/00000441-195801000-00006. PMID 13487586. S2CID 762067.
- ^ Cohen WD, Higano N, Robinson RW (June 1958). "Serum lipid and estrogenic effect of manvene, a new estrogen analog; comparison with premarin in men with coronary heart disease". Circulation. 17 (6): 1035–1040. doi:10.1161/01.CIR.17.6.1035. PMID 13547367.
- ^ Spencer H, Kabakow B, Samachson J, Laszlo D (December 1959). "Metabolic effects of mytatrienediol in man". The Journal of Clinical Endocrinology and Metabolism. 19 (12): 1581–1596. doi:10.1210/jcem-19-12-1581. PMID 13833251.
- ^ Marmorston J, Magdison O, Kuzma O, Moore FJ (September 1960). "Estrogen therapy in men with myocardial infarction. Side-effects with increasing dosage and time". JAMA. 174 (3): 241–244. doi:10.1001/jama.1960.03030030021004. PMID 14421377.
- ^ Bedford PD, Lodge B (December 1959). "Toxic effects of a new antilipaemic oestrogenic steroid (manvene)". Journal of the American Geriatrics Society. 7 (12): 911–915. doi:10.1111/j.1532-5415.1959.tb00364.x. PMID 13798190. S2CID 45355299.
- ^ Kabakow B, Spencer H (June 1960). "Effects of mytatrienediol in multiple myeloma, metastatic bone disease, and osteoporosis". Archives of Internal Medicine. 105 (6): 905–913. doi:10.1001/archinte.1960.00270180083011. PMID 14408289.
