Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal.[1] This triple bond consists of a σ-bond and two π-bonds.[2] The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.[3]
YouTube Encyclopedic
-
1/3Views:6301 7934 850
-
BONDING IN METAL CARBYNE (M≡C) COMPLEXES | Nature of Bonding | CSIR NET | M Sc. Chemistry
-
Transition metal complex (ZEISE SALT) | Alkene comPlex | msc 2nd semester notes |
-
Carbynes – The new piece of chemistry's Lego (The Suero Group)
Transcription
Synthesis
Transition metal carbyne complexes are most common for the early transition metals, especially niobium, tantalum, molybdenum, tungsten, and rhenium. They can also have low-valence metals as well as high-valence metals.

The first Fischer carbyne complex was reported in 1973.[4] Two years later in 1975, the first "Schrock carbyne" was reported.[5]
Many high-valent carbyne complexes have since been prepared, often by dehydrohalogenation of carbene complexes. Alternatively, amino-substituted carbyne ligands sometimes form upon protonation of electron-rich isonitrile complexes. Similarly, O-protonation of μ3-CO ligands in clusters gives hydroxycarbyne complexes. Vinyl ligands have been shown to rearrange into carbyne ligands. Addition of electrophiles to vinylidene ligands also affords carbyne complexes.[3]
Bridging alkylidyne ligands in cluster compounds

Some metal carbynes dimerize to give dimetallacyclobutadienes. In these complexes, the carbyne ligand serves as a bridging ligand.
Several cluster-bound carbyne complexes are known, typically with CO ligands. These compounds do not feature MC triple bonds; instead the carbyne carbon is tetrahedral. Tricobalt derivatives are prepared by treating cobalt carbonyl with haloforms:[6]
- 2 HCBr3 + 9⁄2 Co2(CO)8 → 2 HCCo3(CO)9 + 18 CO + 3 CoBr2
Structure
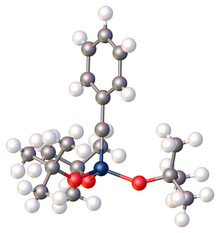
Monomeric metal carbyne complexes exhibit fairly linear M–C–R linkages according to X-ray crystallography. The M–C distances are typically shorter than the M–C bonds found in metal carbenes. The bond angle is generally between 170° and 180°[8] Analogous to Fischer and Schrock carbenes; Fischer and Schrock carbynes are also known. Fischer carbynes usually have lower oxidation state metals and the ligands are π-accepting/electron-withdrawing ligands. Schrock carbynes on the other hand typically have higher oxidation state metals and electron-donating/anionic ligands. In a Fischer carbyne the C-carbyne exhibits electrophilic behavior while Schrock carbynes display nucleophilic reactivity on the carbyne carbon[9] Carbyne complexes have also been characterized by many methods including infrared Spectroscopy, Raman spectroscopy.[10] Bond lengths, bond angles and structures can be inferred from these and other analytical techniques.
Metal carbyne complexes also exhibit a large trans effect, where the ligand opposite the carbyne is typically labile.
Reactions and applications
Hexa(tert-butoxy)ditungsten(III) is a catalyst for alkyne metathesis.[11] The catalytic cycle involves an carbyne intermediate.[12]
Some carbyne complexes react with electrophiles at C-carbyne followed by association of the anion. The net reaction gives a transition metal carbene complex:
- LnM≡CR + HX → Ln(X)M=CHR
These complexes can also undergo photochemical reactions.
In some carbyne complexes, coupling of the carbyne ligand to a carbonyl is observed. Protonation of the carbyne carbon and conversion of the carbyne ligand into a π-allyl.[13]
Main group analogue
A sulfur-based main group analog of a carbyne complex has been prepared by Seppalt and coworkers.[14] The compound, trifluoro(2,2,2-trifluoroethylidyne)-λ6-sulfurane, F3C–C≡SF3, prepared by dehydrofluorination of F3C–CH=SF4 or F3C–CH2–SF5, is an unstable gas that readily undergoes dimerization to form trans-(CF3)(SF3)C=C(CF3)(SF3) at above –50 °C.
Further reading
- Mayr, A.; Bastos, C. M. (1992). Coupling Reactions of Terminal Two-Faced π Ligands and Related Cleavage Reactions. Progress in Inorganic Chemistry. Vol. 40. pp. 1–98. doi:10.1002/9780470166413.ch1. ISBN 9780470166413.
{{cite book}}:|journal=ignored (help)
References
- ^ Cui, Mingxu; Jia, Guochen (2022-07-20). "Organometallic Chemistry of Transition Metal Alkylidyne Complexes Centered at Metathesis Reactions". Journal of the American Chemical Society. 144 (28): 12546–12566. doi:10.1021/jacs.2c01192. ISSN 0002-7863. PMID 35793547.
- ^ Kim, Heesook P.; Angelici, Robert J. (1987). Transition Metal Complexes with Terminal Carbyne Ligands. Advances in Organometallic Chemistry. Vol. 27. pp. 51–111. doi:10.1016/S0065-3055(08)60026-X. ISBN 9780120311279.
{{cite book}}:|journal=ignored (help) - ^ a b Elschenbroich, C. (2006). Organometallics. Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ^ Fischer, E. O.; Kreis, G.; Kreiter, C. G.; Muller, J.; Huttner, G.; Lorenz, H. (1973). "trans-Halogeno-alkyl(aryl)carbin-tetracarbonyl-Komplexe von Chrom, Molybdän und Wolfram–Ein neuer Verbindungstyp mit Übergangsmetall-Kohlenstoff-Dreifachbindung" [trans-Halogenoalkyl(aryl)carbynetetracarbonyl complexes of chromium, molybdenum and tungsten–A new type of compound with a transition metal–carbon triple bond]. Angew. Chem. 85 (14): 618–620. Bibcode:1973AngCh..85..618F. doi:10.1002/ange.19730851407.
- ^ Guggenberger, L. J.; Schrock, R. R. (1975). "Tantalum carbyne complex". J. Am. Chem. Soc. 97 (10): 2935. doi:10.1021/ja00843a072.
- ^ Seyferth, Dietmar; Nestle, Mara O.; Hallgren, John S. (2007). "μ3 -Alkylidyne-Tris(Trigarbonylcobalt) Compounds: Organocobalt Cluster Complexes". Inorganic Syntheses. Vol. 20. pp. 224 224–226]. doi:10.1002/9780470132517.ch52. ISBN 9780470132517.
{{cite book}}:|journal=ignored (help) - ^ Cotton, F. Albert; Schwotzer, Willi; Shamshoum, Edwar S. (1985). "Further studies of the reactions of ditungsten hexa-t- butoxide with acetylenes. Isolation and characterization of WO(OCMe3)4(THF), W3(OCMe3)5(μ-O)(μ-CC3H7)O2 and W(CPh)(OCMe3)3". Journal of Organometallic Chemistry. 296 (1–2): 55–68. doi:10.1016/0022-328X(85)80338-7.
- ^ Spessard, Gary O.; Miessler, Gary L. (2015). Organometallic Chemistry (2nd ed.). Oxford University Press. pp. 439–449. ISBN 9780199342679.
- ^ Crabtree, R. H. (2014). The Organometallic Chemistry of the Transition Metals (6th ed.). New York, NY: Wiley. pp. 290–315. ISBN 9781118138076.
- ^ Kreißl, F. R. (5 December 2012). Transition Metal Carbyne Complexes. Springer. ISBN 9789401047289.
- ^ Fürstner, Alois (2021-09-29). "The Ascent of Alkyne Metathesis to Strategy-Level Status". Journal of the American Chemical Society. 143 (38): 15538–15555. doi:10.1021/jacs.1c08040. ISSN 0002-7863. PMC 8485352. PMID 34519486.
- ^ Listemann, Mark L.; Schrock, Richard R. (1985). "Multiple metal carbon Bonds. 35. A General Route to tri-tert-Butoxytungsten Alkylidyne complexes. Scission of Acetylenes by Ditungsten Hexa-tert-butoxide". Organometallics. 4: 74–83. doi:10.1021/om00120a014.
- ^ Kingsbury, K. B.; Carter, J. D.; McElwee-White, L. (1990). "Formation of cyclopentenone upon photo-oxidation of the cyclopropyl (c-C3H5) carbyne complex [(η5-C5H5){P(OMe)3}(CO)W≡C(c-C3H5)]". J. Chem. Soc., Chem. Commun. 1990 (8): 624–625. doi:10.1039/C39900000624.
- ^ Poetter, Brigitte; Seppelt, Konrad; Simon, Arndt; Peters, Eva Maria; Hettich, Bernhard (February 1985). "Trifluoroethylidynesulfur trifluoride, CF3C.tplbond.SF3, and its dimer". Journal of the American Chemical Society. 107 (4): 980–985. doi:10.1021/ja00290a038. ISSN 0002-7863.
