| |
| Names | |
|---|---|
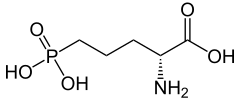
| Preferred IUPAC name
(2R)-2-Amino-5-phosphonopentanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.150.904 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12NO5P | |
| Molar mass | 197.13 g/mol |
| Appearance | white solid |
| Density | 1.529 g/mL |
| Boiling point | 482.1 °C (899.8 °F; 755.2 K) |
| Ammonium hydroxide, 50 mg/mL | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
AP5 (also known as APV, (2R)-amino-5-phosphonovaleric acid, or (2R)-amino-5-phosphonopentanoate) is a chemical compound used as a biochemical tool to study various cellular processes. It is a selective NMDA receptor antagonist that competitively inhibits the ligand (glutamate) binding site of NMDA receptors.[1] AP5 blocks NMDA receptors in micromolar concentrations (~50 μM).
AP5 blocks the cellular analog of classical conditioning in the sea slug Aplysia californica, and has similar effects on Aplysia long-term potentiation (LTP), since NMDA receptors are required for both.[2] It is sometimes used in conjunction with the calcium chelator BAPTA to determine whether NMDARs are required for a particular cellular process. AP5/APV has also been used to study NMDAR-dependent LTP in the mammalian hippocampus.[3]
In general, AP5 is very fast-acting within in vitro preparations, and can block NMDA receptor action at a reasonably small concentration. The active isomer of AP5 is considered to be the D configuration, although many preparations are available as a racemic mixture of D- and L-isomers. It is useful to isolate the action of other glutamate receptors in the brain, i.e., AMPA and kainate receptors.
AP5 can block the conversion of a silent synapse to an active one, since this conversion is NMDA receptor-dependent.
See also
References
- ^ Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. Journal of Neuroscience. 1989 Sep;9(9):3040-57. PMID 2552039
- ^ Cellular Analog of Differential Classical Conditioning in Aplysia: Disruption by the NMDA Receptor Antagonist DL-2-Amino-5-Phosphonovalerate
- ^ Gustafsson B., Wigström H., Abraham W.C., and Huang Y.Y. Long-Term Potentiation in the Hippocampus Using Depolarizing Current Pulses as the Conditioning Stimulus to Single Volley Synaptic Potentials. Journal of Neuroscience. 1987 March;7(3):774-780
External links
- Laube, B; Hirai H, Sturgess M, Betz H, and Kuhse J (1997). "Molecular determinants of antagonists discrimination by NMDA receptor subunits: Analysis of the glutamate binding site on the NR2B subunit". Neuron 18 (3): 493–503. doi:10.1016/S0896-6273(00)81249-0. PMID 9115742.
