| |
| Names | |
|---|---|
| IUPAC name
Magnesium diglutamate(1−)
| |
| Systematic IUPAC name
Magnesium bis(4-amino-4-carboxybutanoate) | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.038.542 |
| E number | E625 (flavour enhancer) |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H16MgN2O8 | |
| Molar mass | 316.549 g·mol−1 |
| Melting point | Tetrahydrate: 130 to 135 °C (266 to 275 °F; 403 to 408 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
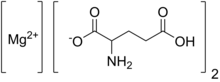
Magnesium diglutamate is a compound with formula Mg(C5H8NO4)2. It is a magnesium acid salt of glutamic acid.
It has the E number E625 and is used in foods as a flavor enhancer.
YouTube Encyclopedic
-
1/1Views:67 229
-
The Dangers of MSG - Part 4 'Avoiding the MSG Threat' (Flavor Enhancers E621 side effects)
Transcription
References
- ^ L-Glutamic acid hemimagnesium salt tetrahydrate at Sigma-Aldrich
